(1-甲基己烷-1,3,5-三基)三苯 | 17293-59-3
分子结构分类
中文名称
(1-甲基己烷-1,3,5-三基)三苯
中文别名
——
英文名称
1,3,5-Triphenylhexan
英文别名
1,3,5-Triphenylhexane;1,5-diphenylhexan-3-ylbenzene
CAS
17293-59-3
化学式
C24H26
mdl
——
分子量
314.47
InChiKey
SLSYKXXGNBFGEK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:156-159 °C(Press: 0.01 Torr)
-
密度:1.0104 g/cm3
-
保留指数:2606.6
计算性质
-
辛醇/水分配系数(LogP):7.3
-
重原子数:24
-
可旋转键数:7
-
环数:3.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-methyl-1,3-diphenyl-pentane 106060-50-8 C18H22 238.373 仲丁基苯 2-butylbenzene 135-98-8 C10H14 134.221 1,3-联苯-1-丁酮 1,3-diphenylbutane-1-one 1533-20-6 C16H16O 224.302
反应信息
-
作为产物:描述:参考文献:名称:Sodium-catalyzed Side Chain Aralkylation of Alkylbenzenes with Styrene1摘要:DOI:10.1021/ja01555a029
文献信息
-
Process for introduction of styrenes in side chain of substituted申请人:Idemitsu Kosan Company Limited公开号:US04703124A1公开(公告)日:1987-10-27A process for introduction of styrenes in the side chain of substituted aromatic compounds, said side chain containing at least one hydrogen atom in the .alpha.-position thereof, is disclosed, comprising reacting the styrenes and the substituted aromatic compounds in the presence of (A) an alkali metal and (B) a compound containing a benzyloxy group or alkyl and/or aryl-substituted benzyloxy group. Use of (A) and (B) as a reaction accelerator permits to introduce the styrenes in the side chain of the substituted aromatic compounds in high yield and selectivity.
-
Regiochemistry of the Styrene Insertion with CH<sub>2</sub>-Bridged <i>a</i><i>nsa</i>-Zirconocene-Based Catalysts作者:Lorella Izzo、Mariagrazia Napoli、Leone OlivaDOI:10.1021/ma034818r日期:2003.12.1eMethylenebis(indenyl)zirconium dichloride substituted in C(3), activated by methylalumoxane, is able to give polystyrene and ethylene-styrene copolymers. In this study hydrooligomers, whose structure, determined by C-13 NMR and GC-MS techniques, gives information about the regiochemistry and the stereochemistry of styrene insertion, have been purposefully prepared. The regiochemistry of the styrene insertion is related to the encumbrance of substituents in C(3). rac-[Methylene-(3-R-1-indenyl)(2)]ZrCl2 with R = H, CH3, or CH2CH3 induces a prevailingly secondary styrene insertion into the zirconium-carbon bond. With increasing the substituent's steric hindrance (R = CH(CH3)(2)), regiochemistry inversion occurs and the primary insertion becomes prevailing. The analysis of ethylene-styrene copolymers obtained in the presence of the different catalysts allows confirming the correlation between regiochemistry and comonomers' reactivity. Besides, also the stereospecificity can be evaluated from the structure of the hydrotrimers, when the insertion is primary. Whereas the isospecificity in the absence of substituents (secondary insertion) and in the presence of the tert-butyl substituent (primary insertion) is well-known, a surprising syndiospecificity is observed when the indenyl ligand bears the isopropyl substituent in C(3).
-
<b>Thiabenzene. II. Rearrangement of 1-Alkyl-2,4,6-triphenylthiabenzenes to 2- and 4-Alkyltriphenylthiopyrans</b>作者:George. Suld、Charles C. PriceDOI:10.1021/ja00870a018日期:1962.6
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
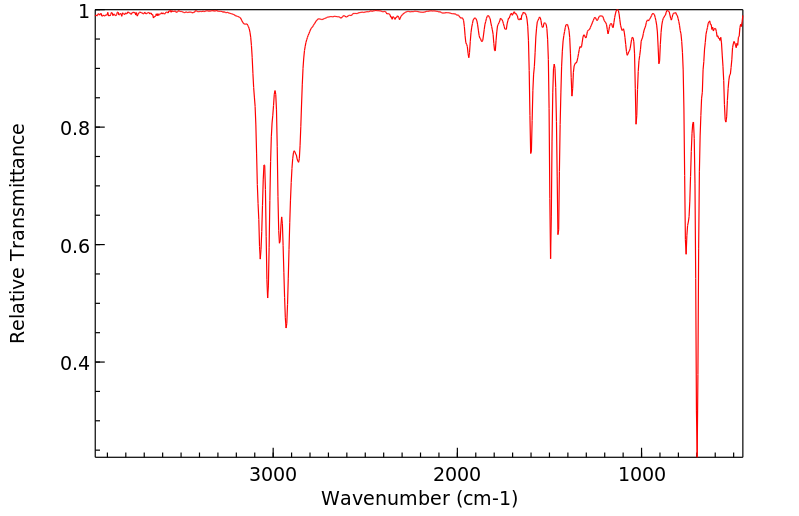
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2R,3R)-4-(蒽-9-基)-3-(叔丁基)-2-甲基-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
黄花菜木脂素B
黄皮树碱
黄小檗碱
鹅掌楸碱
鬼臼酸哌啶基腙氮氧自由基
鬼臼酸
鬼臼脂毒酮
鬼臼毒醇
鬼臼毒素-4-O-葡萄糖苷
鬼臼毒素
鬼臼毒素
高阿莫灵碱
顺式-1,4-二苯基-2-甲氧基-2-丁烯-1,4-二酮
阿罗莫灵
防己诺林碱
防己索林
金不换萘酚
金不换素
里立脂素B二甲醚
连翘脂素
达卡他韦杂质7
赤式-愈创木基甘油-BETA-O-4'-二氢松柏醇
襄五脂素
表鬼臼毒素乙醚
表芝麻素单儿茶酚
表去甲络石甙元
蔚瑞昆森
蒿脂麻木质体
蒽,9,10-二[4-(2,2-二苯基乙烯基)苯基]-
落叶松树脂醇二甲醚
落叶松树脂醇
萘并[2,3-d]-1,3-二噁唑-5(6H)-酮,8-(1,3-苯并二噁唑-5-基)-7,8-二氢-6,7-二甲基-,(6R,7S,8R)-rel-(-)-
萘并[2,3-c]呋喃-1,3-二酮,6-甲氧基-4-(4-甲氧苯基)-
萘并[2,3-c]呋喃-1(3H)-酮,7-羟基-4-(3-甲氧苯基)-
萘并[2,3-c]呋喃-1(3H)-酮,4-(2-氟苯基)-7-(苯基甲氧基)-
萘并[1,2-d]-1,3-二噁唑,9-(1,3-苯并二噁唑-5-基)-6,7-二氢-7,8-二甲基-,(7S)-
萘,1-氯-2-乙基-3-甲基-4-苯基-
荜澄茄素
荜澄茄内酯
荛花酚
苯雌酚二甲醚
苯雌酚
苯酚,5-[2-(3-羟基苯基)乙基]-3-[4-[2-(3-羟基苯基)乙基]苯氧基]-2-甲氧基-
苯酚,4,4'-(四氢-3,4-二甲基-2,5-呋喃二基)二[2-甲氧基-,(2R,3R,4S,5R)-rel-(-)-(9CI)
苯甲醇,4-羟基-3-甲氧基-a-[(1S)-1-[2-甲氧基-4-(1E)-1-丙烯-1-基苯氧基]乙基]-,(aS)-
苯甲醇,3,4-二甲氧基-a-[1-[2-甲氧基-4-(2-丙烯基)苯氧基]乙基]-
苯甲醇,3,4-二甲氧基-a-[1-(2-甲氧基苯氧基)乙基]-
苯甲醇,3,4-二甲氧基-a-[1-(2-甲氧基苯氧基)乙基]-
苯氧基-9苯基-10蒽