7,9-二甲基苯并吖啶 | 963-89-3
中文名称
7,9-二甲基苯并吖啶
中文别名
7,9-二甲基苯并(C)氮杂蒽
英文名称
7,9-dimethylbenz[c]acridine
英文别名
7,9-dimethylbenzo[c]acridine
CAS
963-89-3
化学式
C19H15N
mdl
——
分子量
257.335
InChiKey
HEFJMRLDXHSXEP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:157-161 °C (lit.)
-
沸点:300 °C/10 mmHg (lit.)
-
密度:0.9329 (rough estimate)
-
物理描述:7,9-dimethylbenz(c)acridine is a light yellow powder. (NTP, 1992)
-
溶解度:less than 1 mg/mL at 64° F (NTP, 1992)
-
保留指数:2769;434;438.32
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:20
-
可旋转键数:0
-
环数:4.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:12.9
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:T
-
危险类别码:R20/21/22,R45,R36/37/38,R46
-
海关编码:2933990090
-
WGK Germany:3
反应信息
-
作为反应物:描述:7,9-二甲基苯并吖啶 在 sodium hypochlorite 、 sodium phosphate buffer 、 tetra-tert-butylammonium hydrogen sulfate 作用下, 以 氯仿 为溶剂, 反应 1.5h, 以24%的产率得到7,9-dimethylbenz[c]acridine 5,6-oxide参考文献:名称:Identification of Hepatic Metabolites of Two Highly Carcinogenic Polycyclic Aza-Aromatic Compounds, 7,9-Dimethylbenz[c]acridine and 7,10-Dimethylbenz[c]acridine摘要:The hepatic microsomal metabolites of the highly carcinogenic dimethylbenzacridines, 7,9-dimethylbenz[c]acridine (7,9-DMBAC), and 7,10-dimethylbenz[c]acridine (7,10-DMBAC) were obtained with preparations from 3-methylcholanthrene-pretreated rats. Metabolites were separated by reversed-phase HPLC and characterized using UV spectral data and chemical ionization-mass spectrometry after trimethylsilylation and GC. Comparisons with products formed in the presence of the epoxide hydrolase inhibitor, 1,1,1-trichloropropane 2,3-oxide and with those formed from the three synthetic alcohol derivatives of each parent compound, aided the assignment of firm or tentative structures to 16 products from 7,9-DMBAC found in 22 reversed-phase chromatographic peaks, and for 17 products of 7,10-DMBAC found in 19 chromatographic peaks. The more abundant metabolites were derived from oxidation of the methyl groups. Other metabolites were dihydrodiols, epoxides, phenols and secondary metabolites. The 9-methyl group prevented dihydrodiol formation at the 8,9-position from 7,9-DMBAC, and for each carcinogen, the 3,4-dihydrodiol was formed. As well, 3,4-dihydrodiols of methyl oxidized compounds were found.DOI:10.1021/tx00044a003
-
作为产物:描述:参考文献:名称:Buu-Hoi; Lecocq, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1944, vol. 218, p. 792摘要:DOI:
文献信息
-
Acridine–Isoxazole and Acridine–Azirine Hybrids: Synthesis, Photochemical Transformations in the UV/Visible Radiation Boundary Region, and Anticancer Activity作者:Ekaterina E. Galenko、Mikhail S. Novikov、Alexander S. Bunev、Alexander F. KhlebnikovDOI:10.3390/molecules29071538日期:——Easy-to-handle N-hydroxyacridinecarbimidoyl chloride hydrochlorides were synthesized as convenient nitrile oxide precursors in the preparation of 3-(acridin-9/2-yl)isoxazole derivatives via 1,3-dipolar cycloaddition with terminal alkynes, 1,1-dichloroethene, and acrylonitrile. Azirines with an acridin-9/2-yl substituent attached directly or via the 1,2,3-triazole linker to the azirine C2 were also通过与末端炔烃、1,1-二氯乙烯的 1,3-偶极环加成反应制备 3-(吖啶-9/2-基)异恶唑衍生物,合成了易于处理的 N-羟基吖啶碳酰氯盐酸盐作为方便的氧化腈前体和丙烯腈。还合成了带有吖啶-9/2-基取代基的氮丙啶,该取代基直接或通过1,2,3-三唑连接体连接至氮丙啶C2。尽管吖啶-吖丙啶杂化物的三元环在320-420 nm处有长波吸收,但其三元环被发现对紫外/可见边界区域的辐射具有抵抗力,这表明吖啶部分不能用作天线转移光能,从氮丙啶生成腈叶立德,用于光点击环加成。在蓝光照射下,在 C9-C3 或 C2-C3 原子上连接的吖啶-异恶唑杂化物经历了氢供体溶剂的添加,例如甲苯、邻二甲苯、均三甲苯、4-氯甲苯、THF、1,4-将二恶烷或甲基叔丁基醚(MTBE)加入到吖啶体系中,以良好的收率得到相应的9-取代吖啶。合成的吖啶-吖啶、吖啶-异恶唑和吖啶-异恶唑杂化物对所有测试的癌细胞系(HCT 1
-
364. The relative reactivity of aromatic double bonds. Part III. The relation between double-bond character and the velocity of addition of osmium tetroxide作者:G. M. BadgerDOI:10.1039/jr9500001809日期:——
-
AVNIR D.; BLUM J., J. HETEROCYCL. CHEM. <JHTC-AD>, 1976, 13, NO 3, 619-621作者:AVNIR D.、 BLUM J.DOI:——日期:——
-
PHOTOCATALYTIC DEGRADATION OF SUGAR申请人:EMPIRE TECHNOLOGY DEVELOPMENT LLC公开号:US20160008783A1公开(公告)日:2016-01-14Systems having at least one photonic antenna molecule and at least one catalyst for degrading a sugar to degradation products using light energy are disclosed. Also disclosed are the devices and methods that use the systems for photocatalytically degrading a sugar into degradation products.
-
[EN] PHOTOCATALYTIC DEGRADATION OF SUGAR<br/>[FR] DÉCOMPOSITION PHOTOCATALYTIQUE D'UN SUCRE申请人:EMPIRE TECHNOLOGY DEV LLC公开号:WO2014126647A1公开(公告)日:2014-08-21Systems having at least one photonic antenna molecule and at least one catalyst for degrading a sugar to degradation products using light energy are disclosed. Also disclosed are the devices and methods that use the systems for photocatalytically degrading a sugar into degradation products.
表征谱图
-
氢谱1HNMR
-
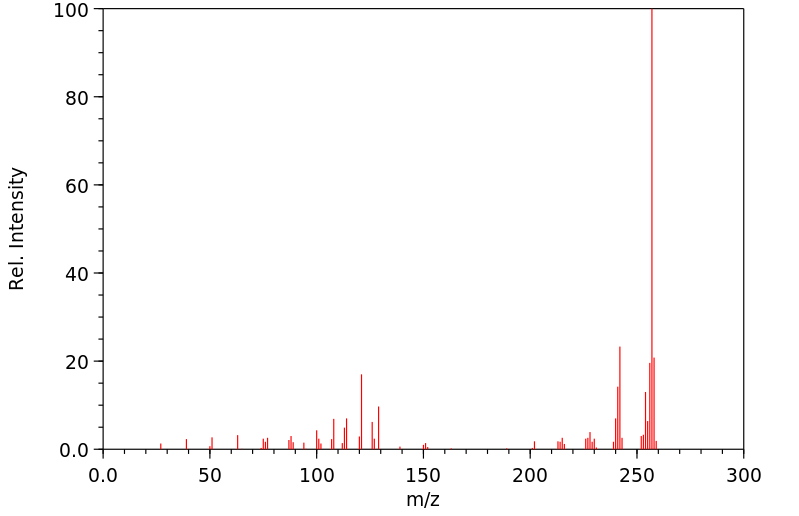
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43