4-甲基-1-庚醇 | 817-91-4
中文名称
4-甲基-1-庚醇
中文别名
4-甲基庚醇
英文名称
4-methyl-1-heptanol
英文别名
4-methylheptan-1-ol;4-Methyl-heptanol-(1);4-methylheptanol
CAS
817-91-4
化学式
C8H18O
mdl
——
分子量
130.23
InChiKey
LLUQZGDMUIMPTC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-61.15°C (estimate)
-
沸点:182.7°C
-
密度:0.8065
-
介电常数:4.5300000000000002
-
LogP:2.721 (est)
-
保留指数:1973
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-甲基庚酸 4-Methyl-heptansaeure 3302-03-2 C8H16O2 144.214 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 6-Methylnonan-3-ol 174323-02-5 C10H22O 158.284 4-甲基庚酸 4-Methyl-heptansaeure 3302-03-2 C8H16O2 144.214 —— (R)-7-Methyl-decanoic acid 475477-77-1 C11H22O2 186.294 —— 7-methyldecanoic acid 116496-50-5 C11H22O2 186.294
反应信息
-
作为反应物:参考文献:名称:Sex pheromone of caddisflyHesperophylax occidentalis (Banks) (Trichoptera: Limnephilidae)摘要:The main component of the sex pheromone of the caddisfly Hesperophylax occidentalis (Banks) (Trichoptera: Limnephilidae) was identified as 6-methylnonan-3-one (enantiomeric composition has not yet been determined). Extracts of adult females elicited strong electroantennogram (EAG) responses from adult male antennae, but elicited significantly smaller EAG responses from adult female antennae. Extracts of adult males did not elicit appreciable EAG responses from antennae of either sex. Racemic 6-methylnonan-3-one was synthesized and elicited EAG responses from male antennae as strong as those obtained with female extracts. In field tests with baited sticky traps near lakes and streams, traps baited with synthetic racemic 6-methylnonan-3-one caught significantly more males than control traps. Female adults contained approximately 1 mu g of 6-methylnonan-3-one per individual. Related ketones and alcohols of other chain lengths were also tentatively identified, being present in tiny amounts in female extracts. Extraction of different body parts showed that 6-methylnonan-3-one occurs only in a region near the intersegmental membrane between the fourth and fifth abdominal sternites of the female (no discrete glands were observed). Extracts of males did not contain 6-methylnonan-3-one, nor did pupae of either sex.DOI:10.1007/bf02040203
-
作为产物:参考文献:名称:Do enzymes recognise remotely located stereocentres? Highly enantioselective Candida rugosa lipase-catalysed esterification of the 2- to 8-methyldecanoic acids摘要:Several racemic methyl decanoic acids have been synthesised and Successfully resolved in esterification with 1-hexadecanol at a(w)=0.8 in cyclohexane using immobilised Candida rugosa lipase (CRL) as the catalyst. The enantiomeric ratios (E=2.8-68) obtained were surprisingly high even when the methyl group was as remotely located as in 8-methyldecanoic acid (E=25). Interestingly, the lipase shows enantiopreference for the S-enantiomer when the methyl group is located on even numbered carbons i.e. for the 2-,4-,6- and 8-methyldecanoic acids and to the R-enantiomer when the methyl group is located on uneven numbered carbons i.e. for the 3-,5- and 7-methyldecanoic acids. (C) 2002 Published by Elsevier Science Ltd.DOI:10.1016/s0957-4166(02)00172-6
文献信息
-
[EN] TGR5 AGONISTS<br/>[FR] AGONISTES DE TGR5申请人:EXELIXIS INC公开号:WO2011071565A1公开(公告)日:2011-06-16TGR5 agonists of structural formula VIII(Q), wherein X, R1, R2, and R5 are defined in the specification, pharmaceutically acceptable salts thereof, compositions thereof, and use of the compounds and compositions for treating diseases. The invention also comprises use of the compounds in and for the manufacture of medicaments, particularly for treating diseases.TGR5激动剂的结构式VIII(Q),其中X、R1、R2和R5在规范中定义,其药用盐、组合物以及用于治疗疾病的化合物和组合物的使用。该发明还包括在制药品中使用这些化合物以及用于制造药物,特别是用于治疗疾病。
-
[EN] TRIAZOLE AND IMIDAZOLE DERIVATIVES FOR USE AS TGR5 AGONISTS IN THE TREATMENT OF DIABETES AND OBESITY<br/>[FR] DÉRIVÉS DE TRIAZOLE ET D'IMIDAZOLE DESTINÉS À ÊTRE UTILISÉS EN TANT QU'AGONISTES DE TGR5 DANS LE TRAITEMENT DU DIABÈTE ET DE L'OBÉSITÉ申请人:EXELIXIS INC公开号:WO2010093845A1公开(公告)日:2010-08-19The present invention comprises TGR5 agonists of structural formula I, wherein X, R1, R2, and R5 are defined herein, as well as N-oxides of them and pharmaceutically acceptable salts thereof. The invention further comprises composition comprising the compounds, N-oxides, and/or pharmaceutically acceptable salts thereof. The invention also comprises use of the compounds and compositions for treating diseases in which TGR5 is a mediator or is implicated. The invention also comprises use of the compounds in and for the manufacture of medicaments, particularly for treating diseases in which TGR5 is a mediator or is implicated.本发明包括结构式I的TGR5激动剂,其中X、R1、R2和R5在此处定义,以及它们的N-氧化物和其药学上可接受的盐。该发明还包括包含这些化合物、N-氧化物和/或其药学上可接受的盐的组合物。该发明还包括利用这些化合物和组合物治疗TGR5是介质或涉及的疾病。该发明还包括利用这些化合物制造药物,特别是用于治疗TGR5是介质或涉及的疾病。
-
[EN] PHTALAZINE DERIVATIVES AS JAK1 INHIBITORS<br/>[FR] DÉRIVÉS DE LA PHTALAZINE COMME INHIBITEURS DE JAK1申请人:EXELIXIS INC公开号:WO2012037132A1公开(公告)日:2012-03-22JAK1 inhibitors of structural formula (I), wherein Ar1, Ar2, Q, W, X, Y, and Z are defined in the specification, pharmaceutically acceptable salts thereof, compositions thereof, and use of the compounds and compositions for treating diseases. The invention also comprises use of the compounds in and for the manufacture of medicaments, particularly for treating diseases.JAK1抑制剂的结构公式(I),其中Ar1、Ar2、Q、W、X、Y和Z在说明书中定义,药用可接受的盐,它们的组合物以及用于治疗疾病的化合物和组合物的用途。本发明还包括在制造药物中的用途,特别是在治疗疾病中的用途。
-
PROCESSES FOR THE SYNTHESIS OF CHIRAL 1-ALKANOLS申请人:PURDUE RESEARCH FOUNDATION公开号:US20160332940A1公开(公告)日:2016-11-17The invention relates to highly enantioselective processes for the synthesis of chiral 1-alkanols via Zr-catalyzed asymmetric carboalumination of alkenes.这项发明涉及通过Zr催化的不对称烯烃碳硼烷化反应合成手性1-烷醇的高对映选择性过程。
-
PROCESS FOR DOUBLE CARBONYLATION OF ALLYL ALCOHOLS TO CORRESPONDING DIESTERS申请人:EVONIK DEGUSSA GMBH公开号:US20170174610A1公开(公告)日:2017-06-22The invention relates to a process for doubly carbonylating allyl alcohols to the corresponding diesters, wherein a linear or branched allyl alcohol is reacted with a linear or branched alkanol (alcohol) with supply of CO and in the presence of a catalytic system composed of a palladium complex and at least one organic phosphorus ligand and in the presence of a hydrogen halide selected from HCl, HBr and HI.
表征谱图
-
氢谱1HNMR
-
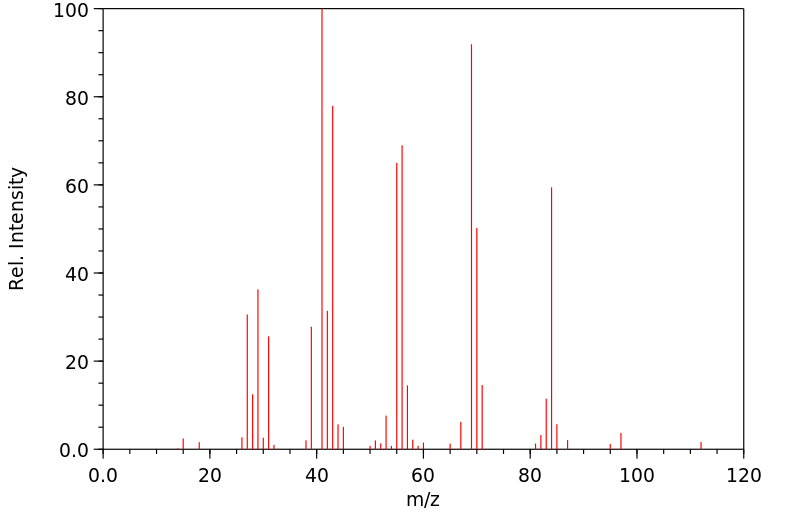
质谱MS
-
碳谱13CNMR
-
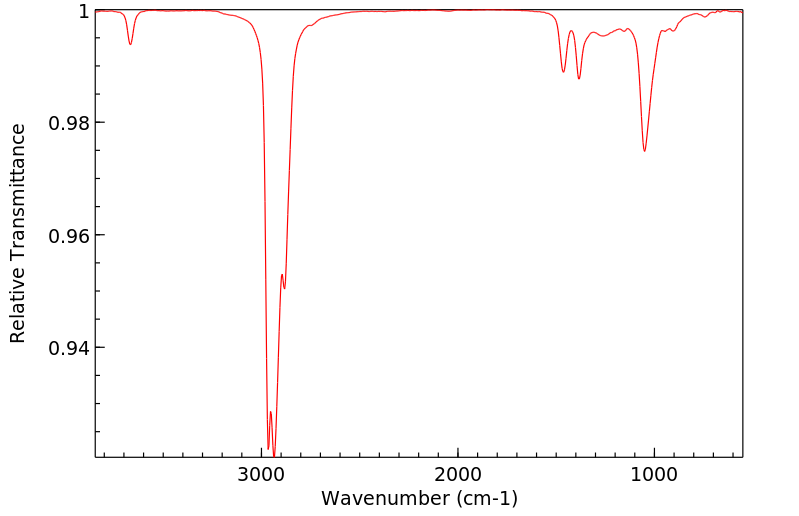
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯