2-异硫代氰酰丙酸乙酯 | 39574-16-8
中文名称
2-异硫代氰酰丙酸乙酯
中文别名
L-2-异硫代氰酰基丙酸乙酯
英文名称
ethyl 2-isothiocyanatopropionate
英文别名
2-isothiocyanatopropionic acid ethyl ester;2-Isothiocyan-propionsaeure-ethylester;α-Isothiocyan-propionsaeure-ethylester;ethyl (+/-)-2-isothiocyanatopropanoate;Inakt. (α-Carbaethoxy-aethyl)-isothiocyanat;N-Thiocarbonyl-DL-alanin-aethylester;2-(Isothiocyanato)propionsaeure-ethylester;N-thiocarbonyl-alanine ethyl ester;Thiocarbonyl-dl-alaninaethylester;Ethyl 2-isothiocyanatopropanoate
CAS
39574-16-8
化学式
C6H9NO2S
mdl
——
分子量
159.209
InChiKey
ALJGYASQFZQQJX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:44 °C
-
密度:1,09 g/cm3
-
闪点:63-64°C/1mm
-
保留指数:1141.8
-
稳定性/保质期:
常规情况下不会分解,也没有任何危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:70.8
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:6.1
-
危险品标志:Xi
-
危险类别码:R20/21/22,R36/37/38
-
危险品运输编号:UN 2810
-
海关编码:2930909090
-
包装等级:III
-
危险类别:6.1
-
安全说明:S26,S36/37/39
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 丙氨酸乙酯 Alanine ethyl ester 17344-99-9 C5H11NO2 117.148
反应信息
-
作为反应物:描述:2-异硫代氰酰丙酸乙酯 在 potassium tert-butylate 、 双氧水 、 对甲苯磺酸 作用下, 以 四氢呋喃 、 甲酸 为溶剂, 反应 0.25h, 生成 4-methyl-2-oxo-5c-phenyl-oxazolidine-4r-carboxylic acid ethyl ester参考文献:名称:Hoppe,D.; Follmann,R., Chemische Berichte, 1976, vol. 109, p. 3047 - 3061摘要:DOI:
-
作为产物:描述:参考文献:名称:Johnson; Ticknor, Journal of the American Chemical Society, 1918, vol. 40, p. 645摘要:DOI:
文献信息
-
Enolates of 2-Isothiocyanatocarboxylic Esters: Synthesis of Thiazolo[5,4-d]-thiazole Derivatives and 2-Thioxo-1,3-thiazolidine-4-carboxylates作者:Dariusz Cież、Justyna Kalinowska-TłuścikDOI:10.1055/s-0031-1290825日期:2012.6acids leads to radical coupling followed by cyclization. This cascade reaction gives thiazolo[5,4-d]thiazole derivatives as pure enantiomers. Under similar conditions, 2-methylbutyl esters of 2-isothiocyanatocarboxylic acids undergo intermolecular oxidative dimerization to give mixtures of thiazolo[5,4-d]thiazoles and 2,3-diisothiocyanatosuccinates. Application of the soft enolization technique to摘要 衍生自2-异硫代羧酸薄荷酯的烯醇钛(IV)的氧化二聚作用导致自由基偶联,然后环化。该级联反应得到作为纯对映异构体的噻唑并[5,4- d ]噻唑衍生物。在相似的条件下,2-异硫氰酸根合羧酸的2-甲基丁酯进行分子间氧化二聚生成噻唑并[5,4- d]thiazoles and 2,3-diisothiocyanatosuccinates. Application of the soft enolization technique to dimethyl α,α′-diisothiocyanatodicarboxylic esters gives novel cyclic 1,2-diisothiocyanato-1,2-dicarboxylates. Sodium enolates of 2-isothiocyanatocarboxylates, on the other hand, form
-
An Approach to 2,3-Diaminosuccinic Acid Derivatives—Synthesis of 2-Thioxo-1,3-Imidazolidines by a Mannich Reaction作者:Dariusz Cież、Justyna Kalinowska-Tłuścik、Jakub MarchewkaDOI:10.1071/ch11463日期:——The article deals with a new synthesis of 2-thioxo-1,3-imidazolidine derivatives, masked 2,3-diaminosuccinic acids. The described procedure leads to the designed products by an intermolecular Mannich reaction between aldimine (ethyl iminoacetate) and enolates derived from 2-isothiocyanatocarboxylic esters. The prepared heterocyclic products having two stereogenic centers can be easily separated into
-
Preparation of New Nitrogen-Bridged Heterocycles. 42. Synthesis and the Reaction of Pyridinium<i>N</i>-Ylides Using Bifunctional Ethyl Thiocyanatoacetates作者:Akikazu Kakehi、Suketaka Ito、Yasunobu HashimotoDOI:10.1246/bcsj.69.1769日期:1996.6cycloadditions of some pyridinium (unsymmetrically substituted cyanomethylide)s with dimethyl acetylenedicarboxylate (DMAD) in various solvents afforded only dimethyl 3-cyanoindolizine-1,2-dicarboxylate, except a few examples. On the other hand, the treatment of pyridinium (thiocyanatoaceto)- or (2-thiocyanatopropiono)amidates with a strong base, such as potassium t-butoxide, gave new bicyclic mesoionic compounds各种吡啶鎓(单取代的甲基化物)在硫氰酸乙酯或 2-硫氰酸根合丙酸乙酯中顺利地攻击氰基,以低到中等的产率得到相应的吡啶鎓(取代的氰基甲基化物),而吡啶鎓(未取代的酰胺化物)与在相同的试剂中酯羰基以可观的产率得到吡啶鎓(硫氰酸根合乙酰基)-或(2-硫氰酸根合丙基)酰胺。除了几个例子外,一些吡啶鎓(不对称取代的氰基甲基化物)与乙炔二甲酸二甲酯 (DMAD) 在各种溶剂中的 1,3-偶极环加成仅得到 3-氰基吲哚腙-1,2-二甲酸二甲酯。另一方面,用强碱(如叔丁醇钾)处理吡啶鎓(硫氰基乙酰基)-或(2-硫氰基丙酸基)酰胺,得到了新的双环介离子化合物 N-[2-(1,3,4-噻二唑并[3,2-a]吡啶并)]乙酰胺衍生物,收率适中。N-[1-(2-thiocyanatopyridinio)]aceta的中间体...
-
Titanium(IV)-mediated synthesis of 2,3-diisothiocyanato-succinic acid diesters and 3,6-dithioxo-piperazine derivatives作者:Dariusz CieżDOI:10.1016/j.tet.2007.03.053日期:2007.5Oxidative homocoupling of titanium(IV) enolates of 2-isothiocyanato-carboxylic esters resulted in the synthesis of 2,3-diisothiocyanato-succinic acid diesters. The reactions were carried out using DIPEA/TiCl4 oxidizing system and led to chiral dimers (instead of meso) as main products. Titanium(IV) enolates derived from hindered 2-isothiocyanato-carboxylates did not undergo the oxidative homocoupling
-
Common ligand mimics: thiazolidinediones and rhodanines申请人:Yu Lin公开号:US20050042674A9公开(公告)日:2005-02-24The present invention provides common ligand mimics that act as common ligands for a receptor family. The present invention also provides bi-ligands containing these common ligand mimics. Bi-ligands of the invention provide enhanced affinity and/or selectivity of ligand binding to a receptor or receptor family through the synergistic action of the common ligand mimic and specificity ligand which compose the bi-ligand. The present invention also provides combinatorial libraries containing the common ligand mimics and bi-ligands of the invention. Further, the present invention provides methods for manufacturing the common ligand mimics and bi-ligands of the invention and methods for assaying the combinatorial libraries of the invention.
表征谱图
-
氢谱1HNMR
-
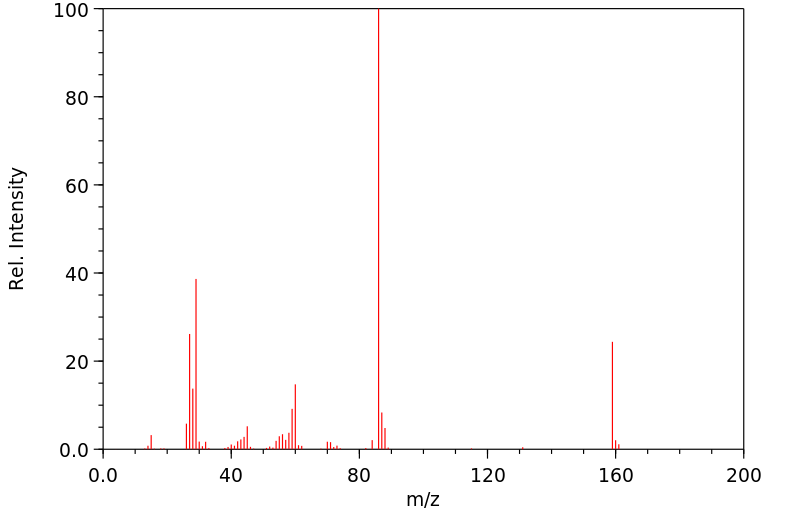
质谱MS
-
碳谱13CNMR
-
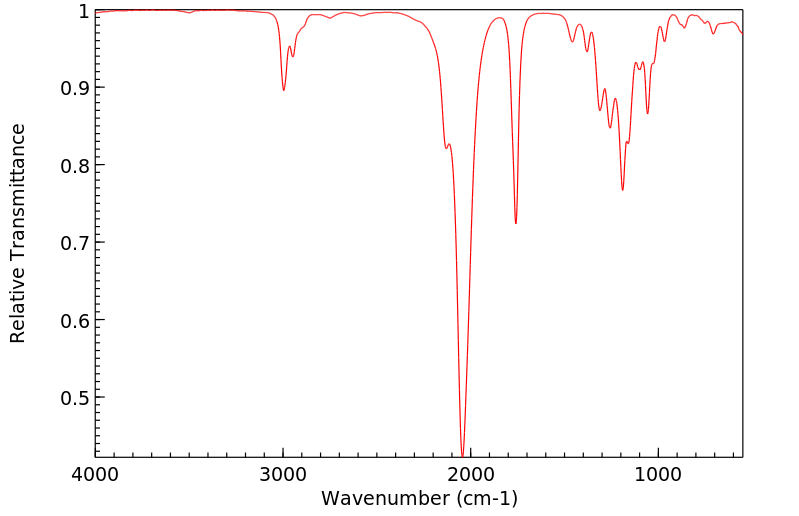
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸