N-甲基-O-苯甲酰基羟胺盐酸盐 | 27130-46-7
中文名称
N-甲基-O-苯甲酰基羟胺盐酸盐
中文别名
N-甲基-O-苯基羟胺盐酸盐;O-苯甲酰基-N-甲基羟胺盐酸盐
英文名称
N-methyl-O-benzoylhydroxylamine hydrochloride
英文别名
O-benzoyl-N-methylhydroxylamine hydrochloride;benzoyloxy(methyl)azanium;chloride
CAS
27130-46-7
化学式
C8H9NO2*ClH
mdl
——
分子量
187.626
InChiKey
FTCDQNQIGUCFSA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:136°C(lit.)
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:38.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
储存条件:室温且干燥
SDS
模块 1. 化学品
1.1 产品标识符
: N-甲基-O-苯基羟胺盐酸盐
产品名称
1.2 鉴别的其他方法
Benzoic acid N-hydroxymethylamine ester hydrochloride
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Benzoic acid N-hydroxymethylamine ester hydrochloride
别名
: C8H10ClNO2
分子式
: 187.62 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
建议的贮存温度: 2 - 8 °C
充气保存 吸湿的.
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 133 °C - 分解
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
电池材料
反应信息
-
作为反应物:描述:N-甲基-O-苯甲酰基羟胺盐酸盐 在 palladium on activated charcoal N-甲基吗啉 、 氢气 、 氯甲酸异丁酯 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 反应 5.5h, 生成 N-[4-(N-benzyloxy-N-methylcarbamoyl)-n-butanoyl]-N6-[N2',N6'-bis(2,3-diacetoxybenzoyl)-L-lysyl]-L-lysine参考文献:名称:New synthetic siderophores and Their β-Lactam conjugates based on diamino acids and dipeptides摘要:Linking of siderophores to antibiotics improves the penetration and therefore increases the antibacterial activity of the antibiotics. We synthesized the acylated catecholates and hydroxamates as siderophore components for antibiotic conjugates to reduce side effects of unprotected catecholate and hydroxamate moieties. In this paper, we report on bis- and tris-catecholates and mixed catecholate hydroxamates based on diamino acids or dipeptides. These compounds were active as siderophores in a growth promotion assay under iron limitation. Most of the conjugates with beta-lactams showed high in vitro activity against Gram-negative bacteria especially Pseudomonas acruginosa, Escherichia coli, Klebsiella pneumoniae, Serratia mareeseems and Stenotrophomonas maltophilia. The compounds with enhanced antibacterial activity use active iron uptake routes to penetrate the bacterial outer membrane barrier, demonstrated by assays with mutants deficient in components of the iron transport system. Correlation between chemical structure and biological activity was studied. (C) 2002 Published by Elsevier Science Ltd.DOI:10.1016/s0968-0896(02)00044-5
-
作为产物:描述:N-(tert-butyloxycarbonyl)-N-methyl-O-benzoyl-hydroxylamine 在 盐酸 作用下, 以 1,4-二氧六环 为溶剂, 反应 2.0h, 以95%的产率得到N-甲基-O-苯甲酰基羟胺盐酸盐参考文献:名称:A General Method for the α-Acyloxylation of Carbonyl Compounds摘要:A simple, one-pot method for the alpha-acyloxylation of carbonyl compounds that proceeds at room temperature in the presence of both moisture and air has been developed. Treatment of a variety of aldehydes and both cyclic and acyclic ketones with N-methyl-O-benzoylhydroxylamine hydrochloride provides the alpha-functionalized product in 69-92% isolated yield. The transformation is tolerant of a wide range of functional groups and, significantly, is reglospecific in the discrimination of secondary over primary centers in the case of nonsymmetrical substrates.DOI:10.1021/ol052474e
-
作为试剂:描述:2,2'-Dimethoxy-[1,1']bi[dibenzofuranyl] 在 三溴化硼 、 N-甲基-O-苯甲酰基羟胺盐酸盐 作用下, 以 二氯甲烷 、 二甲基亚砜 为溶剂, 反应 46.0h, 生成 C24H14O4参考文献:名称:通过限制分子运动增强烷基锁定轴向手性支架的圆偏振发光摘要:通过减少烷基化轴向支架的链长来增加分子内刚性并增强分子间相互作用以限制分子间运动的协同策略对增强圆偏振发光非常有效。不对称因素异常且显着的放大 |克伦|在有机CPL体系中实现了从10 -4到10 -1 的水平。DOI:10.1002/chem.202303667
文献信息
-
NGCYCLOARTANONE DERIVATIVES WITH ANTICANCER ACTIVITY申请人:Long Christophe公开号:US20120077777A1公开(公告)日:2012-03-29The present invention relates to a compound of following formula (I): or to a pharmaceutically acceptable salt thereof, as well as to pharmaceutical compositions including same and to the use thereof as a drug, in particular for treating a proliferative disease such as cancer.本发明涉及以下式(I)的化合物: 或其药学上可接受的盐,以及包括相同的药物组合物和将其用作药物的用途,特别是用于治疗增殖性疾病如癌症。
-
Asymmetric α-oxyacylation of cyclic ketones作者:Deborah A. Smithen、Christopher J. Mathews、Nicholas C. O. TomkinsonDOI:10.1039/c2ob25293g日期:——Reaction of cyclic ketones with chiral N-alkyl-O-acyl hydroxylamines leads to the corresponding α-oxyacylated carbonyl compound in up to 89% ee. The levels of asymmetric induction were influenced by solvent polarity, acid strength and, to a lesser extent, temperature. Increasing the steric bulk around the nitrogen atom of the hydroxylamine reagent led to increased levels of asymmetric induction, which was also found to be detrimental to the yield observed for the transformation. Examination of N- and O-substituents along with substrates revealed the scope and limitations of the procedure.
-
Total synthesis and biological evaluation of (−)-atrop–abyssomicin C作者:Filip Bihelovic、Ivanka Karadzic、Radomir Matovic、Radomir N. SaicicDOI:10.1039/c3ob40692j日期:——Enantioselective synthesis of a marine antibiotic (−)-atrop–abyssomicin C was accomplished in 21 steps, in 1.8% overall yield (4%, based on the recovered starting material). The key steps of the synthesis are the formation of the functionalized cyclohexane core by an organocatalyzed Tsuji–Trost reaction, the formation of a tricyclic spirotetronate unit by a gold-catalyzed reaction sequence and the
-
α-Aroyloxyaldehydes: scope and limitations as alternatives to α-haloaldehydes for NHC-catalysed redox transformations作者:Kenneth B. Ling、Andrew D. SmithDOI:10.1039/c0cc02456b日期:——α-Aroyloxyaldehydes are readily prepared bench stable synthetic intermediates. Their ability to act as α-haloaldehyde surrogates for NHC-promoted redox esterifications and in [4+2] cycloadditions is described.α-芳氧醛是一种容易制备且在桌面上稳定的合成中间体。它们在NHC促进的氧化还原酯化反应以及[4+2]环加成反应中作为α-卤醛的替代物的能力得到了描述。
-
Total Synthesis of the <i>Illicium</i>-Derived Sesquineolignan Simonsol C作者:Jeremy Nugent、Martin G. Banwell、Brett D. SchwartzDOI:10.1021/acs.orglett.6b01799日期:2016.8.5The racemic form of the title natural product 1 has been synthesized by engaging, as a key step, the iodoarene-tethered cyclohexene 22 in an intramolecular Heck reaction to give compound 23. This angularly substituted tetrahydrodibenzo[b,d]furan was elaborated over a further five steps into target (±)-1.
表征谱图
-
氢谱1HNMR
-
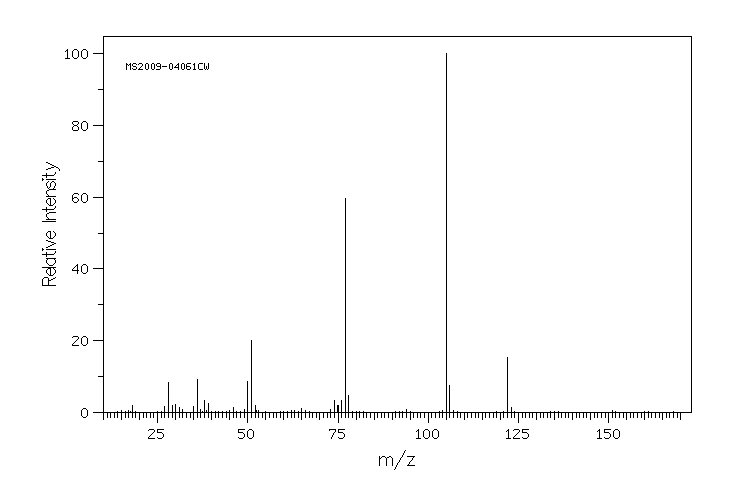
质谱MS
-
碳谱13CNMR
-
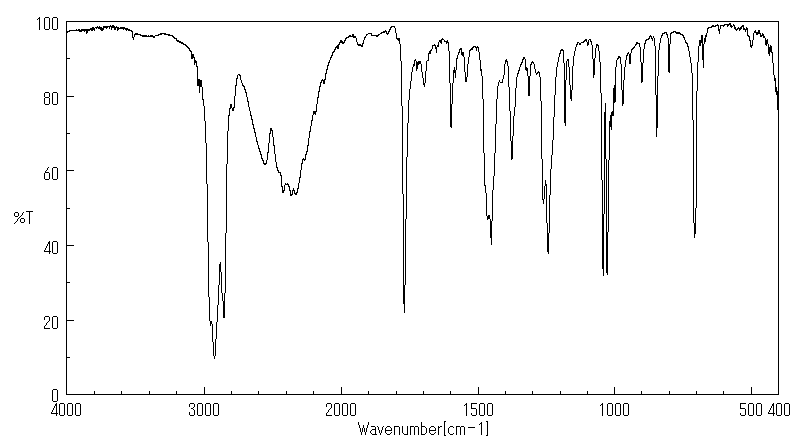
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫