7-十五酮 | 6064-38-6
中文名称
7-十五酮
中文别名
——
英文名称
7-pentadecanone
英文别名
Pentadecan-7-on;pentadecan-7-one
CAS
6064-38-6
化学式
C15H30O
mdl
——
分子量
226.403
InChiKey
VUXXXYMMZBAUSE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:30-31°C
-
沸点:293.03°C (estimate)
-
密度:0.8184 (estimate)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):6.2
-
重原子数:16
-
可旋转键数:12
-
环数:0.0
-
sp3杂化的碳原子比例:0.93
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2914190090
-
危险性防范说明:P210,P280,P370+P378,P403+P235,P501
-
危险性描述:H227,H315
-
储存条件:常温、避光、存于阴凉干燥处并密封保存。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 庚醛 heptanal 111-71-7 C7H14O 114.188
反应信息
-
作为反应物:参考文献:名称:Schmitz; Sonnenschein; Gruendemann, Journal fur praktische Chemie (Leipzig 1954), 1980, vol. 322, # 2, p. 261 - 272摘要:DOI:
-
作为产物:参考文献:名称:Kharasch; Urry; Kuderna, Journal of Organic Chemistry, 1949, vol. 14, p. 251摘要:DOI:
文献信息
-
Oxidative Cleavage of Alkenes by O<sub>2</sub> with a Non-Heme Manganese Catalyst作者:Zhiliang Huang、Renpeng Guan、Muralidharan Shanmugam、Elliot L. Bennett、Craig M. Robertson、Adam Brookfield、Eric J. L. McInnes、Jianliang XiaoDOI:10.1021/jacs.1c05757日期:2021.7.7however. There are only a small number of known synthetic metal catalysts that allow for the oxidative cleavage of alkenes at an atmospheric pressure of O2, with very few known to catalyze the cleavage of nonactivated alkenes. In this work, we describe a light-driven, Mn-catalyzed protocol for the selective oxidation of alkenes to carbonyls under 1 atm of O2. For the first time, aromatic as well as variousC=C双键与分子氧的氧化裂解产生羰基化合物是化学和药物合成中的一个重要转化。在自然界中,含有第一排过渡金属的酶,特别是血红素和非血红素铁依赖性酶,在环境条件下很容易激活 O 2并以极其精确的方式氧化裂解 C=C 键。然而,该反应对合成化学家来说仍然具有挑战性。只有少数已知的合成金属催化剂允许在 O 2大气压下氧化裂解烯烃,很少有人知道催化未活化烯烃的裂解。在这项工作中,我们描述了一种光驱动、Mn 催化的协议,用于在 1 个大气压的 O 2下将烯烃选择性氧化为羰基化合物。首次使用第一排生物相关金属催化剂,在清洁、温和的条件下,可以将芳香族和各种未活化的脂肪族烯烃氧化成酮和醛。此外,该协议显示出非常好的功能组耐受性。机理研究表明,Mn-oxo 物种,包括不对称的混合价双 (μ-oxo)-Mn(III,IV) 络合物,参与氧化,溶剂甲醇参与 O 2活化,导致oxo 物种的形成。
-
[EN] SUBSTITUTED HETEROARYL COMPOUNDS AND METHODS OF USE<br/>[FR] COMPOSÉS HÉTÉROARYLES SUBSTITUÉS ET MÉTHODES D'UTILISATION申请人:CALITOR SCIENCES LLC公开号:WO2015094803A1公开(公告)日:2015-06-25The present invention provides new heteroaryl compounds, pharmaceutical acceptable salts and formulations thereof useful in preventing, treating or lessening the severity of JAK-mediated diseases. The invention also provides pharmaceutically acceptable compositions comprising such compounds and methods of using the compositions in the treatment of JAK-mediated diseases.本发明提供了新的杂环芳基化合物,其药用盐和制剂在预防、治疗或减轻JAK介导疾病的严重程度方面具有用处。该发明还提供了包括这些化合物的药用组合物以及使用这些组合物治疗JAK介导疾病的方法。
-
Palladium-catalyzed carbonylative cross-coupling reaction of iodoalkanes with 9-alkyl-9-BBN derivatives. A direct and selective synthesis of ketones作者:Tatsuo Ishiyama、Norio Miyaura、Akira Suzuki*DOI:10.1016/0040-4039(91)80445-c日期:1991.11The synthesis of unsymmetrical ketones by means of the palladium-catalyzed carbonylative cross-coupling reaction of 9-alkyl-9-BBN derivatives with iodoalkanes under a carbon monoxide atmosphere is described.
-
Cerium oxide as a catalyst for the ketonization of aldehydes: mechanistic insights and a convenient way to alkanes without the consumption of external hydrogen
-
Chiral Synthesis via Organoboranes. 47. Efficient Synthesis of Unsymmetrical Ketones and Enantiomerically Pure Spiroketals Using (±)-Isopinocampheyldichloroborane作者:Herbert C. Brown、Shekhar V. Kulkarni、Uday S. Racherla、Ulhas P. DhokteDOI:10.1021/jo980989w日期:1998.10.1intermediate was readily converted into the unsymmetrical ketones, R(1)COR(2), in high yields and purity, by an established method. This methodology was successfully applied to the synthesis of enantiomerically pure spiroketals using optically pure TBS ether protected homoallylic alcohols as the alkenes for stepwise hydroboration.
表征谱图
-
氢谱1HNMR
-
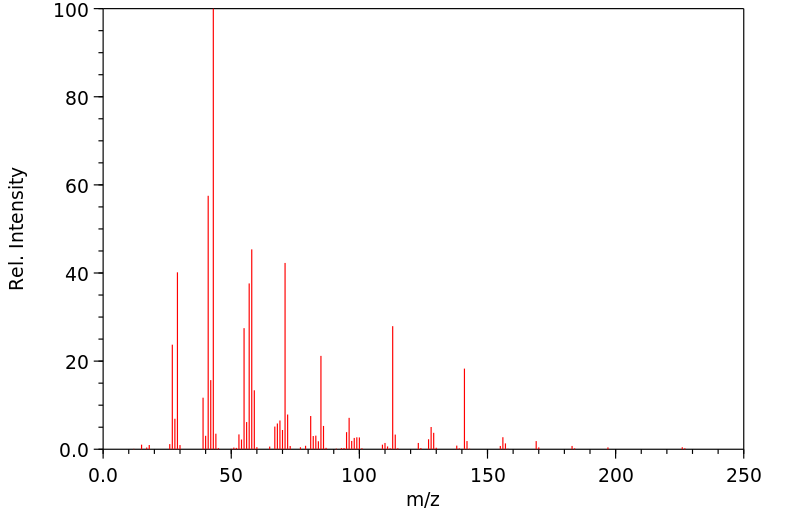
质谱MS
-
碳谱13CNMR
-
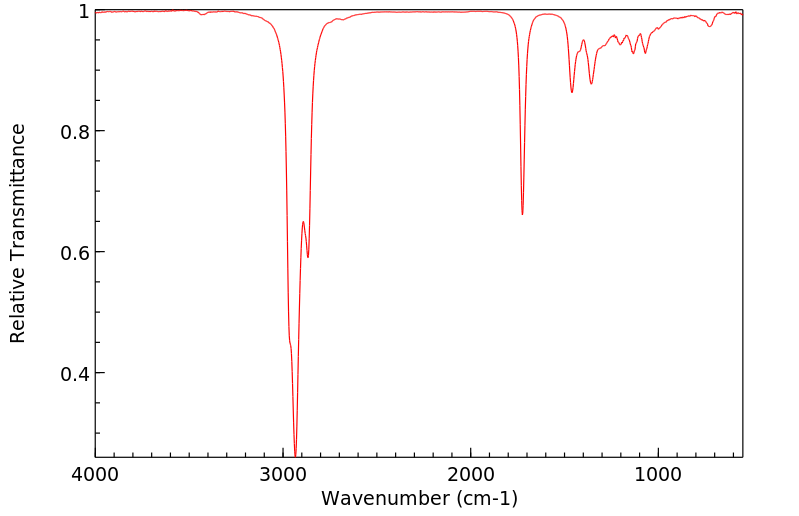
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷