4-乙基-4-庚醇 | 597-90-0
中文名称
4-乙基-4-庚醇
中文别名
——
英文名称
4-Aethyl-heptanol-(4)
英文别名
4-Ethyl-4-heptanol;4-ethylheptan-4-ol
CAS
597-90-0
化学式
C9H20O
mdl
——
分子量
144.257
InChiKey
GNROHGFUVTWFNG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:6.15°C (estimate)
-
沸点:179.5°C
-
密度:0.8310
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-propyl-hex-1-yn-3-ol 682-28-0 C9H16O 140.225
反应信息
-
作为反应物:描述:参考文献:名称:Halse, Journal fur praktische Chemie (Leipzig 1954), 1914, vol. <2>89, p. 452摘要:DOI:
-
作为产物:参考文献:名称:Sung, Annales de Chimie (Cachan, France), 1924, vol. <10>1, p. 364摘要:DOI:
文献信息
-
Iridium-Catalyzed Regioselective Silylation of Secondary Alkyl C–H Bonds for the Synthesis of 1,3-Diols作者:Bijie Li、Matthias Driess、John F. HartwigDOI:10.1021/ja5026479日期:2014.5.7Ir-catalyzed intramolecular silylation of secondary alkyl C-H bonds. (Hydrido)silyl ethers, generated in situ by dehydrogenative coupling of a tertiary or conformationally restricted secondary alcohol with diethylsilane, undergo regioselective silylation at a secondary C-H bond γ to the hydroxyl group. Oxidation of the resulting oxasilolanes in the same vessel generates 1,3-diols. This method provides a strategy
-
[EN] PROCESS<br/>[FR] PROCÉDÉ申请人:PHOSPHAGENICS LTD公开号:WO2018112512A1公开(公告)日:2018-06-28An efficient and commercial phosphorylation process of a complex alcohol, such as secondary and tertiary alcohols, with P4O10 at high temperatures, and a product obtained by the process.
-
Cp2TiCl2-catalyzed grignard reactions. 3. Reactions with esters: Efficient methodology for the synthesis of secondary alcohols and for the reduction of esters to primary alcohols作者:Fumie Sato、Takamasa Jinbo、Masao SatoDOI:10.1016/s0040-4039(00)78990-8日期:1980.1Cp2TiCl2-catalyzed Grignard reactions with esters provide general methodology for preparation of secondary alcohols or for reduction of esters to the corresponding primary alcohols.Cp 2 TiCl 2催化的与酯的格氏反应为制备仲醇或将酯还原为相应的伯醇提供了通用方法。
-
UV/VISIBLE-ABSORBING VINYLIC MONOMERS AND USES THEREOF申请人:Novartis AG公开号:US20170242274A1公开(公告)日:2017-08-24Described herein are UV-absorbing vinylic monomers and their uses in preparing UV-absorbing contact lenses capable of blocking ultra-violet (“UV”) radiation and violet radiation with wavelengths from 380 nm to 440 nm, thereby protecting eyes to some extent from damages caused by UV radiation and potentially from violet radiation.本文描述了UV吸收乙烯基单体及其在制备能够阻挡380纳米至440纳米波长的紫外辐射和紫外辐射的UV吸收隐形眼镜中的用途,从而在一定程度上保护眼睛免受紫外辐射和潜在的紫外辐射造成的损害。
-
[EN] PROCESS FOR RECOVERING 3-METHYLBUT-3-EN-1-OL<br/>[FR] PROCÉDÉ POUR LA RÉCUPÉRATION DE 3-MÉTHYLBUT-3-EN-1-OL申请人:BASF SE公开号:WO2019030386A1公开(公告)日:2019-02-14The present invention relates to a process for recovering 3-methylbut-3-en-1 -ol from a feed stream F1 comprising 3-methylbut-3-en-1 -ol, one or more solvents, water, and isobutene, wherein 3-methylbut-3-en-1 -ol, the one or more solvents and water are separated from isobutene by distillation, the process comprising subjecting the feed stream F1 to distillation conditions in a distillation unit, obtaining a bottoms stream B1 which is enriched in -methylbut-3-en-1 -ol, in the one or more solvents and in water compared to the feed stream F1 subjec The present invention relates to a process for recovering 3-methylbut-3-en-1 -ol from a feed stream F1 comprising 3-methylbut-3-en-1 -ol, one or more solvents, water, and isobutene, wherein 3-methylbut-3-en-1 -ol, the one or more solvents and water are separated from isobutene by distillation, the process comprising subjecting the feed stream F1 to distillation conditions in a distillation unit, obtaining a bottoms stream B1 which is enriched in -methylbut-3-en-1 - ol, in the one or more solvents and in water compared to the feed stream F1 subjected to distillation conditions, and a top stream T1 which is enriched in isobutene, further subjecting the bottoms stream B1 to distillation conditions in a second distillation unit and obtaining a bottoms stream B2 which is enriched in 3-methylbut-3-en-1-ol compared to the bottoms stream B1 and a top stream T2 which is enriched in water compared to the bottoms stream B1, further subjecting the bottoms stream B2 to distillation conditions in a third distillation unit and obtaining a top stream T3 which is enriched in 3-methylbut-3-en-1-ol compared to the bottoms stream B2 and a bottoms stream B3. ted to distillation conditions, and a top stream T1 which is enriched in isobutene, further subjecting the bot- toms stream B1 to distillation conditions in a second distillation unit and obtaining a bottoms stream B2 which is enriched in 3-methylbut-3-en-1-ol compared to the bottoms stream B1 and a top stream T2 which is enriched in water compared to the bottoms stream B1, further subjecting the bottoms stream B2 to distillation conditions in a third distillation unit and obtaining a top stream T3 which is enriched in 3-methylbut-3-en-1-ol compared to the bottoms stream B2 and a bottoms stream B3.本发明涉及一种从含有3-甲基丁-3-烯-1-醇、一种或多种溶剂、水和异丁烯的进料流F1中回收3-甲基丁-3-烯-1-醇的方法,其中通过蒸馏将3-甲基丁-3-烯-1-醇、一种或多种溶剂和水与异丁烯分离,该方法包括将进料流F1在蒸馏单元中进行蒸馏条件处理,获得富含3-甲基丁-3-烯-1-醇、一种或多种溶剂和水的底流B1,相比于经蒸馏条件处理的进料流F1,以及富含异丁烯的顶流T1,进一步将底流B1在第二蒸馏单元中进行蒸馏条件处理,获得富含3-甲基丁-3-烯-1-醇的底流B2,相比于底流B1富含水的顶流T2,进一步将底流B2在第三蒸馏单元中进行蒸馏条件处理,获得富含3-甲基丁-3-烯-1-醇的顶流T3,相比于底流B2富含水的底流B3。
表征谱图
-
氢谱1HNMR
-
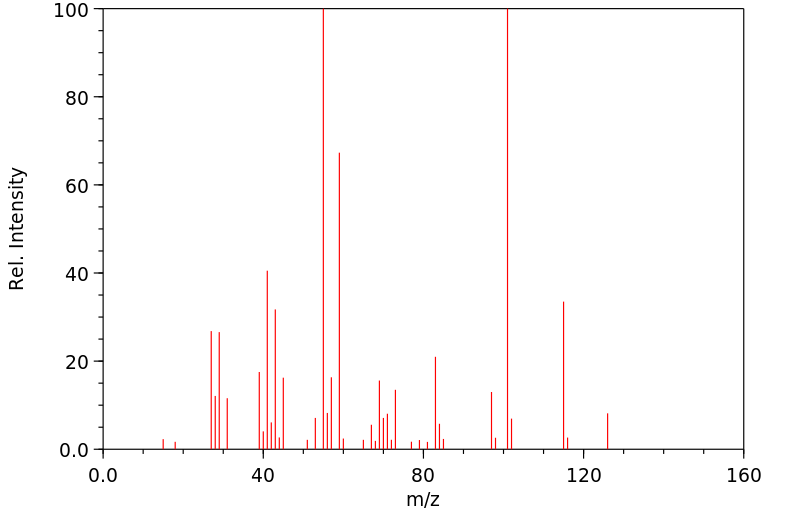
质谱MS
-
碳谱13CNMR
-
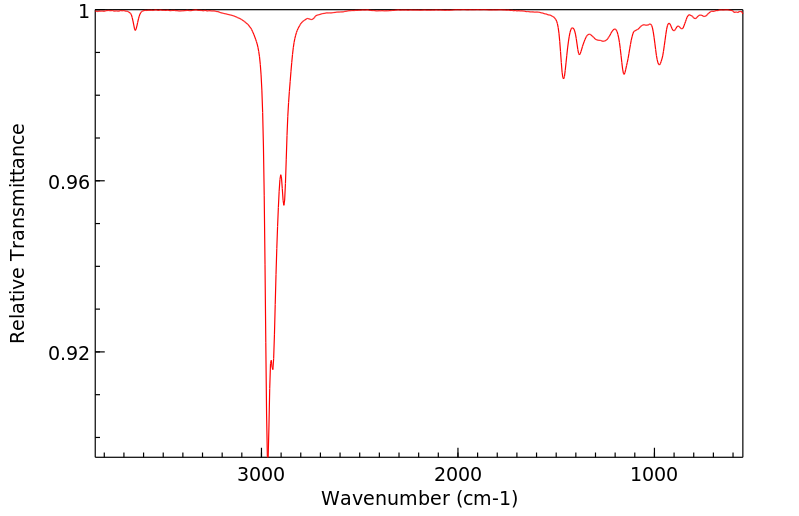
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷