3-乙基-2-甲基-1-戊烯 | 19780-67-7
中文名称
3-乙基-2-甲基-1-戊烯
中文别名
——
英文名称
3-ethyl-2-methyl-pent-2-ene
英文别名
3-Aethyl-2-methyl-pent-2-en;1.1-Dimethyl-2.2-diaethyl-aethylen;3-Isopropyliden-pentan;α.α-Dimethyl-β.β-diaethyl-aethylen;γ-Isopropyliden-pentan;2-Methyl-3-ethyl-2-pentene;3-ethyl-2-methylpent-2-ene
CAS
19780-67-7
化学式
C8H16
mdl
MFCD00060947
分子量
112.215
InChiKey
FQYUGAXHZSQHMU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-103.01°C (estimate)
-
沸点:120.2°C (estimate)
-
密度:0.7350
-
保留指数:791.8;778
计算性质
-
辛醇/水分配系数(LogP):3.8
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2901299090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-2-戊烯 2-methyl-2-pentene 625-27-4 C6H12 84.1613
反应信息
-
作为反应物:描述:参考文献:名称:Radiolytic reductions and oxidations in dimethyl sulfoxide solutions: solvent effects on reactivity of halogen atom complexes摘要:Radiolysis of dimethyl sulfoxide (DMSO) solutions containing various additives was used to achieve clean one-electron reduction of oxidation of solutes. Pulse radiolysis of benzoquinone in DMSO solutions containing acetone and triethylamine permitted conversion of all primary radicals into reducing species. The total yield of reduction in the gamma-radiolysis of methyl viologen solutions was found to be 0.37-mu-mol/J. In the pulse radiolysis of TMPD and triphenylamine in aerated DMSO containing LiCl and/or CCl4, all the primary radicals were converted into oxidizing species and gave a maximum yield of 0.39-mu-mol/J. In the latter systems, oxidation was partly by halogen atom complexes. The reactivity of complexes of DMSO (DMSO.Cl, DMSO.Br) and of halide ions (Br2.-,I2.-) was examined for several organic compounds. DMSO.Cl oxidizes chlorpromazine, triphenylamine, and zinc porphyrin with rate constants of the order of 10(7)-10(8) M-1 s-1, and the rates increase upon addition of CH2Cl2 as well as upon addition of water and formamide. DMSO.Cl also reacts with olefins by addition of Cl to the double bond; the rate constants increase upon increasing the electron-donating properties of the substituents on the double bond. The rate constants for oxidation of chlorpromazine by Br2.- and I2.- increase by more than 2 orders of magnitude upon changing the solvent from DMSO gradually to water. The change was less with acetonitrile/water mixtures, and the difference is probably due to differences in ion solvation.DOI:10.1021/j100187a032
-
作为产物:参考文献:名称:Grigorowitsch; Pawlow, Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1891, vol. 23, p. 169摘要:DOI:
文献信息
-
Stetter,H.; Tresper,E., Chemische Berichte, 1971, vol. 104, p. 71 - 74作者:Stetter,H.、Tresper,E.DOI:——日期:——
-
Buck et al., Journal of the Institute of Petroleum, 1949, vol. 35, p. 648作者:Buck et al.DOI:——日期:——
-
Halomethyl-metal compounds. XIII. Preparation of gem-difluorocyclopropanes by iodide ion-induced CF2 transfer from trimethyl(trifluoromethyl)tin作者:Dietmar Seyferth、Hadwig Dertouzos、Reiichi Suzuki、Jeffrey Y. P. MuiDOI:10.1021/jo01285a011日期:1967.10
-
Localization of excitation energy in chemically activated systems. 3-Ethyl-2-methyl-2-pentyl radicals作者:M. C. Flowers、B. S. RabinovitchDOI:10.1021/j100250a003日期:1985.2
-
Olefin synthesis by vanadium(V)-induced oxidative decarboxylation-deoxygenation of 3-hydroxy carboxylic acids作者:Ingrid K. Meier、Jeffrey SchwartzDOI:10.1021/jo00308a021日期:1990.10
表征谱图
-
氢谱1HNMR
-
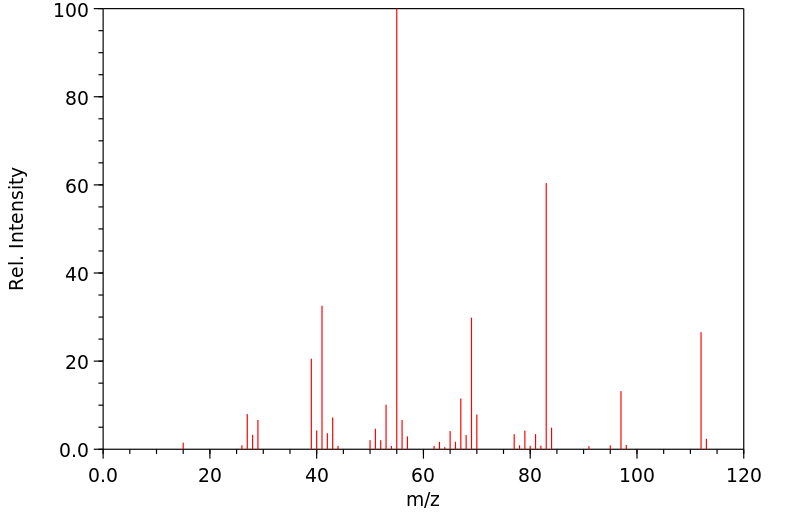
质谱MS
-
碳谱13CNMR
-
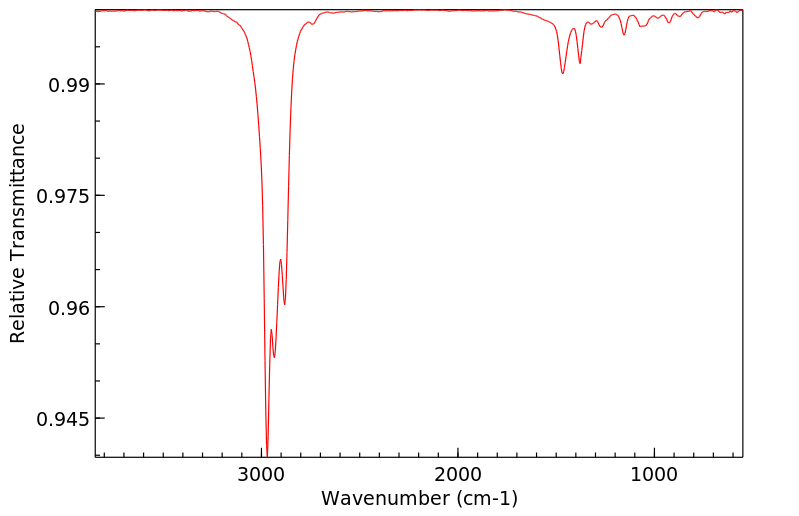
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-