3-(2-nitro-benzoyl)-dihydro-furan-2-one | 52459-80-0
中文名称
——
中文别名
——
英文名称
3-(2-nitro-benzoyl)-dihydro-furan-2-one
英文别名
α-(2-nitrobenzoyl)-γ-butyrolactone;dihydro-3-(o-nitrobenzoyl)-2(3H)-furanone;2-<2-Nitro-benzoyl>-γ-butyrolacton;3-(2-Nitro-benzoyl)-2-oxo-tetrahydrofuran;2(3H)-Furanone, dihydro-3-(2-nitrobenzoyl)-;3-(2-nitrobenzoyl)oxolan-2-one
CAS
52459-80-0
化学式
C11H9NO5
mdl
——
分子量
235.196
InChiKey
HJJMZFWWAYITCI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75-76 °C
-
沸点:475.8±35.0 °C(Predicted)
-
密度:1.424±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:17
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.27
-
拓扑面积:89.2
-
氢给体数:0
-
氢受体数:5
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Herbicide intermediate o-nitrophenyl cyclopropyl ketone and a method for摘要:提供了o-硝基苯基环丙基酮,这是制造作物选择性除草剂1-{[o-(环丙基甲酰)苯基]磺胺基}-3-(4,6-二甲氧基-2-嘧啶基)脲的关键中间体,并提供了一种从二氢-3-乙酰基-2(3H)-呋喃酮和o-硝基苯甲酰卤制备该酮的方法。公开号:US05364968A1
-
作为产物:参考文献:名称:CNS depressant .gamma.-(secondary amino)-ortho-nitro-butyrophenones摘要:公式为:##STR1## 其中 R 是氢原子或氟原子,W 是氧原子或乙二氧基或乙二硫基,Z 是某种次级氨基团,这些化合物可作为中枢神经系统抑制剂,可以通过下式化合物的反应制备:##STR2## 其中 X 是卤素原子,R 和 W 如上所定义,与下式次级胺基的化合物反应:H--Z 其中 Z 如上所定义,如果 W 是乙二氧基或乙二硫基,则可以选择性地进行水解,然后还可以还原得到下式化合物:##STR3## 其中 R 和 Z 如上所定义,这些化合物可作为抗精神病和/或镇痛剂。公开号:US04075346A1
-
作为试剂:描述:、 α-乙酰基-γ-丁内酯 、 2-硝基苯甲酰氯 、 水 在 氮 、 3-(2-nitro-benzoyl)-dihydro-furan-2-one 、 甲苯 作用下, 以 甲苯 为溶剂, 反应 4.75h, 以to give the title product as a toluene solution, 875 g, 18% desired product by LC analysis (67% yield)的产率得到3-(2-nitro-benzoyl)-dihydro-furan-2-one参考文献:名称:Herbicide intermediate o-nitrophenyl cyclopropyl ketone and a method for摘要:提供了o-硝基苯基环丙酮,是生产农作物选择性除草剂1-{[o-(环丙基羰基)苯基]磺酰胺}-3-(4,6-二甲氧基-2-嘧啶基)脲的关键中间体,以及从二氢-3-乙酰基-2(3H)-呋喃酮和o-硝基苯甲酰卤制备该酮的方法。公开号:US05364968A1
文献信息
-
Nitrene. Part XIII. Novel conversion of 2-nitrophenyl substituted butyrolactones into indoles with triethyl phosphite作者:Tetsuji Kametani、Frank F. Ebetino、Keiichiro FukumotoDOI:10.1039/p19740000861日期:——Reductive cyclization of α-(2-hydroxyethyl)-β-methoxy-o-nitrocinnamic acid γ-lactone (4) with triethyl phosphite produced 3,4-dihydro-5-methoxy[1,3]oxazino[3,4-a]indol-1-one (6), a new heterocyclic ring system. Reduction of 2-o-nitrobenzoyl-γ-butyrolactone (3) yielded 4,5-dihydro-1′H-spiro[furan-3,2′-indole]-2,3′-dione (8) as the major product, in addition to a trace amount of the 5-ethoxy-analogue
-
Herbicide intermediate o-nitrophenyl cyclopropyl ketone and a method for the preparation thereof申请人:AMERICAN CYANAMID COMPANY公开号:EP0577945A1公开(公告)日:1994-01-12There is provided o-nitrophenyl cyclopropyl ketone, a key intermediate in the manufacture of the crop-selective, herbicidal agent 1-[o-(cyclopropylcarbonyl)phenyl]sulfamoyl}-3-(4,6-dimethoxy-2-pyrimidinyl)urea and a method for the preparation of said ketone from dihydro-3-acetyl-2(3H)-furanone and o-nitrobenzoyl halide.
-
4-Hydroxy-2'-nitrobutyrophenone and tetrahydro-2-(o-nitrophenyl)-2-furanol useful as intermediates in the preparation of a crop-selective herbicide申请人:AMERICAN CYANAMID COMPANY公开号:EP0604705A1公开(公告)日:1994-07-06There are provided 4-Hydroxy-2'-nitrobutyrophenone and tetrahydro-2-(o-nitrophenyl)-2-furanol and mixtures thereof, important intermediates in the preparation of the crop-selective herbicidal agent 1-[o-(cyclopropylcarbonyl)phenyl]sulfamoyl}-3-(4,6-dimethoxy-2-pyrimidinyl)urea. Also provided is a method for the preparation of 4-halo-2'-nitrobutyrophenone, useful as an intermediate in the preparation of 1-[o-(cyclopropylcarbonyl)phenyl]sulfamoyl}-3-(4,6-dimethoxy-2-pyrimidinyl)urea.
-
JPH06199742A申请人:——公开号:JPH06199742A公开(公告)日:1994-07-19
-
JPH0672967A申请人:——公开号:JPH0672967A公开(公告)日:1994-03-15
表征谱图
-
氢谱1HNMR
-
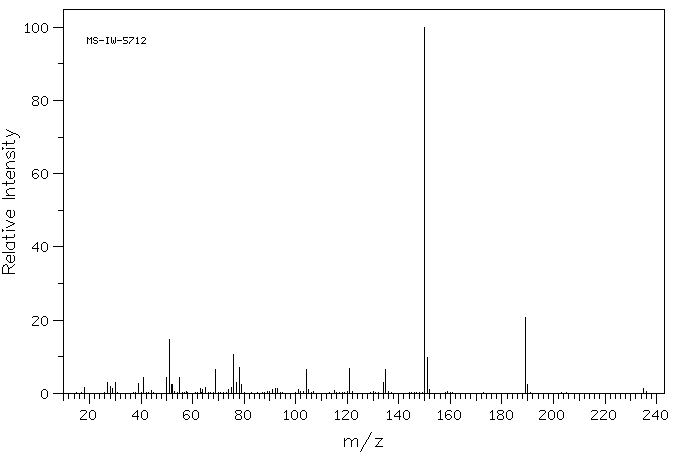
质谱MS
-
碳谱13CNMR
-
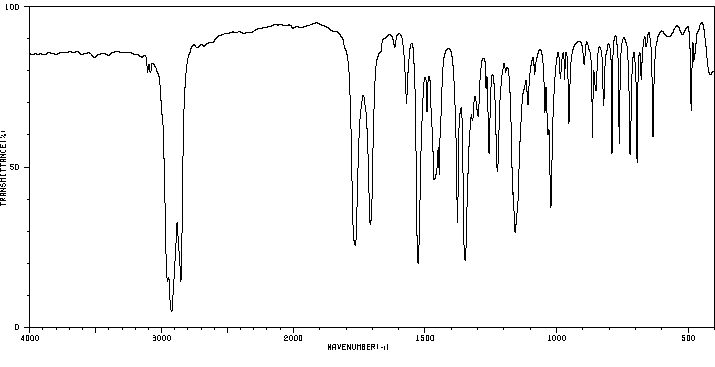
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷