(S)-(+)-S-甲基-S-苯亚磺酰亚胺 | 33903-50-3
中文名称
(S)-(+)-S-甲基-S-苯亚磺酰亚胺
中文别名
(S)-(+)-s-甲基-s-苯基磺脒;(R)-(+)-S-甲基-S-苯亚磺酰亚胺;(S)-(+)-S-甲基-S-苯亚砜亚胺
英文名称
(S)-S-methyl-S-phenylsulfoximine
英文别名
S-methyl-S-phenyl-sulfoximine;(S)-imino(methyl)(phenyl)-λ6-sulfanone;(S)-methyl phenyl sulfoximine;methyl phenyl sulfoximine;(S)-(+)-S-Methyl-S-phenylsulfoximine;imino-methyl-oxo-phenyl-λ6-sulfane
CAS
33903-50-3;60933-65-5;4381-25-3
化学式
C7H9NOS
mdl
——
分子量
155.221
InChiKey
YFYIDTVGWCYSEO-JTQLQIEISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:30-32°C
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:49.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R22
-
海关编码:2925290090
-
WGK Germany:3
-
危险性防范说明:P264,P270,P301+P312,P330,P501
-
危险性描述:H302
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 (甲基亚砜亚胺基)苯 S-methyl-S-phenylsulfoximine 4381-25-3 C7H9NOS 155.221 甲基苯基亚砜 racemic methyl phenyl sulfoxide 1193-82-4 C7H8OS 140.206 —— (S)-methylphenylsulfoxide 18453-46-8 C7H8OS 140.206 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N,S-dimethyl-S-phenylsulfoximine 33993-53-2 C8H11NOS 169.247 —— (+)-(S)-N-(2-Hydroxyethyl)-S-methyl-3-phenylsulfoximine 210552-35-5 C9H13NO2S 199.274 —— (S)-methylphenylsulfoxide 18453-46-8 C7H8OS 140.206 甲基苯基亚砜 racemic methyl phenyl sulfoxide 1193-82-4 C7H8OS 140.206 —— N-trimethylsilyl-S-methyl-S-phenylsulfoximine 152983-29-4 C10H17NOSSi 227.403 —— (+)-(S)-N-(3-Hydroxypropyl)-S-methyl-S-phenylsulfoximine 210552-36-6 C10H15NO2S 213.301 苯甲砜 Methyl phenyl sulfone 3112-85-4 C7H8O2S 156.205
反应信息
-
作为反应物:描述:参考文献:名称:A Method for the Conversion of Sulfoximines to Sulfones: Application to Polymer-Bound Sulfoximines and to the Synthesis of Chiral Sulfones摘要:Reaction of N-alkyl, N-aryl, and N-H sulfoximines with m-chloroperbenzoic acid cleanly gives the corresponding sulfones in high yield. In the case of the cleavage of N-alkyl and N-arylsulfoximines, formation of the corresponding nitroso compounds as the other reaction product was proven. Starting from enantio- and diastereopure sulfoximines, a number of chiral sulfones, including the axially chiral sulfone 6 and the sulfonyl-functionalized homoallylic alcohol 8, have been prepared. Reaction of the enantiopure sulfoximine 30 with Merrifield resin gave the polymer-bound sulfoximine 32. Oxidative cleavage of 32 afforded the sulfone 16 in high yield. Deprotonation of the sulfoximine resin 32 and reaction of Li-32 with benzaldehyde and propanal furnished the beta-hydroxysulfoximine resins 33a and 33b, respectively. Oxidative cleavage of 33a and 33b readily afforded the beta-hydroxy sulfones 14a and 14b, respectively.DOI:10.1002/(sici)1099-0690(200004)2000:8<1457::aid-ejoc1457>3.0.co;2-g
-
作为产物:描述:甲基苯基亚砜 在 sodium azide 、 硫酸 、 (1S)-(+)-10-camphorsulfonic acid 作用下, 以 氯仿 为溶剂, 生成 (S)-(+)-S-甲基-S-苯亚磺酰亚胺参考文献:名称:通过亚砜基yl,醛和烯酮的反应不对称合成γ-内酯摘要:提出了一种通过对映体富集的亚砜基磺酸盐,醛和烯酮的反应对映选择性合成γ-内酯的方法。使对映体富集(98%ee)的氨基ulf酸叶立德与各种醛(芳族和脂族)和双取代的烯酮反应,导致形成α,β-取代的γ-内酯,具有中等至非常好的非对映选择性(dr 95:5),对映体过量最多为ee的79%。在富含对映体的氨基亚砜基ox与异丁醛和各种烷基芳基烯酮的反应中观察到最佳对映选择性水平。DOI:10.1016/j.tetlet.2014.05.130
文献信息
-
[EN] SULFOXIMINE SUBSTITUTED PYRROLOTRIAZINES FOR PHARMACEUTICAL COMPOSITIONS<br/>[FR] PYRROLOTRIAZINES À SUBSTITUTION SULFOXIMINE POUR COMPOSITIONS PHARMACEUTIQUES申请人:BOEHRINGER INGELHEIM INT公开号:WO2015091156A1公开(公告)日:2015-06-25This invention relates to novel sulfoximine substituted pyrrolotriazine derivatives of formula wherein Ar, R1 and R2 are as defined in the description and claims, and their use as MNK1 (MNK1 a or MNK1 b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1 a or MNK1 b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
N-이미도일 설폭시민 유도체 및 이의 제조 방법申请人:KNU-Industry Cooperation Foundation 강원대학교산학협력단(220040088571) BRN ▼221-82-10213公开号:KR20170109274A公开(公告)日:2017-09-29본 발명은 리간드와 카이랄 보조제로 사용되는 중요한 구조의 성질을 띄고있어 다양한 연구분야에서 이용가능한 신규의 N-이미도일 설폭시민 유도체 및 이의 제조방법에 관한 것으로, 보다 상세하게는 구리 촉매 존재 하에 알카인 유도체, 아자이드 유도체 및 N-설폭시민 유도체를 반응시켜 설폭시민의 질소 원자에 탄소-질소 결합 작용기가 도입된 N-이미도일 설폭시민 유도체를 효율적으로 제조하는 방법 및 이에 따라 제조된 N-이미도일 설폭시민 유도체에 관한 것이다.
-
Asymmetric intramolecular Diels-Alder reactions of sulfoximine-activated trienes作者:Donald Craig、Neil J. Geach、Christopher J. Pearson、Alexandra M.Z. Slawin、Andrew J.P. White、David J. WilliamsDOI:10.1016/0040-4020(95)00267-c日期:1995.5A series of N-substituted sulfoximidoyl-1,6,8-nonatrienes and 1,7,9-decatrienes were synthesised and subjected to thermal intramolecular Diels-Alder (IMDA) reactions to give diastereomeric mixtures of substituted bicyclo[4.3.0]nonanes and -[4.4.0]decanes. The reactions showed varying selectivities. Endo/exo selectivity was interpreted in terms of a combination of steric factors and the asynchronous
-
Sulfoximines: A Reusable Directing Group for Chemo- and Regioselective ortho CH Oxidation of Arenes作者:M. Ramu Yadav、Raja K. Rit、Akhila K. SahooDOI:10.1002/chem.201200092日期:2012.4.27Sulfoximines direct: A new protocol for the chemo‐ and regioselective ortho CH acetoxylation of arenes in N‐benzoylated sulfoximines is reported. The sulfoximine directing group is easily detached from the CH oxidation product through acid‐promoted hydrolysis, isolated, and reused (see scheme). The meta‐substituted phenols are synthesized following this strategy and the stereointegrity of the sulfoximine
-
Metal-Free, Phosphonium Salt-Mediated Sulfoximination of Azine <i>N</i>-Oxides: Approach for the Synthesis of <i>N</i>-Azine Sulfoximines作者:Sravan Kumar Aithagani、Mukesh Kumar、Mahipal Yadav、Ram A. Vishwakarma、Parvinder Pal SinghDOI:10.1021/acs.joc.6b00593日期:2016.7.15method for the synthesis of N-azine sulfoximines by the nucleophilic substitution of azine N-oxides with NH-sulfoximines. The present method works at room temperature with wide functional group compatibility and gives several unprecedented N-azine sulfoximines. The reaction conditions were also found suitable with enantiopure substrates and furnished products without any racemization. It also finds
表征谱图
-
氢谱1HNMR
-
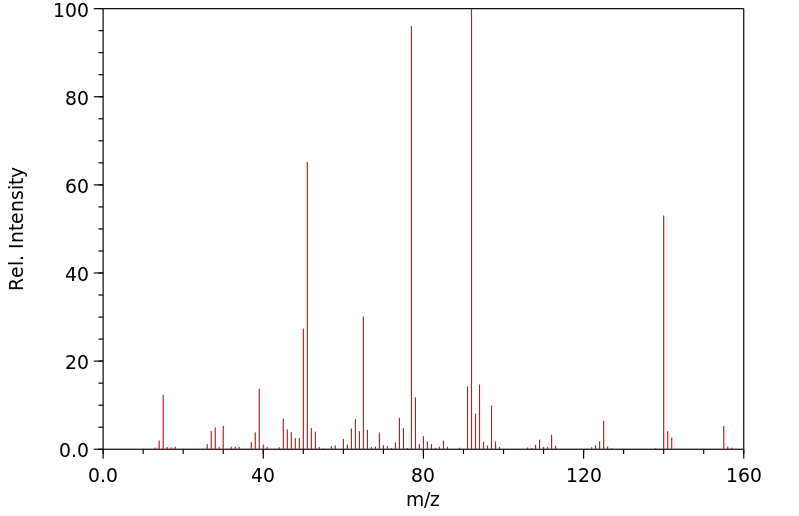
质谱MS
-
碳谱13CNMR
-
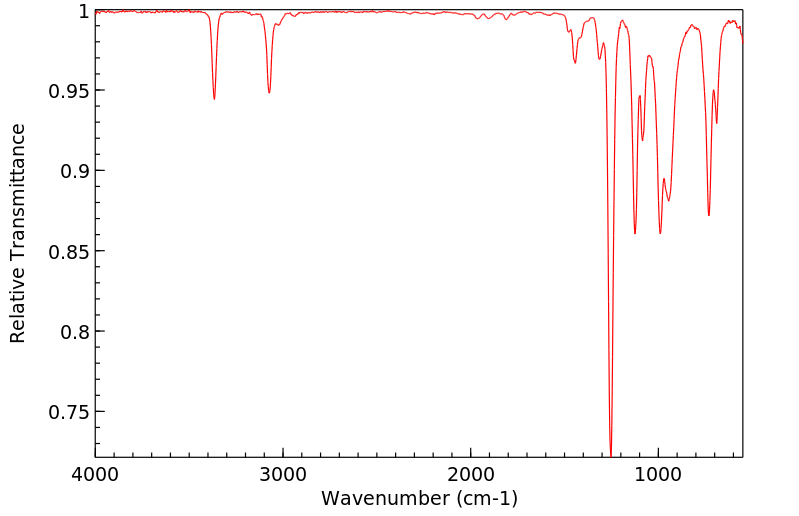
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫