4-甲氧基苯甲砜 | 3517-90-6
中文名称
4-甲氧基苯甲砜
中文别名
4-甲氧基苯基甲基砜;4-甲氧基苯急家基砜
英文名称
p-anisyl methyl sulfone
英文别名
1-methoxy-4-(methylsulfonyl)benzene;4-methoxyphenyl methyl sulfone;p-methoxyphenyl methyl sulfone;1-methanesulfonyl-4-methoxybenzene;4-methylsulfonylanisole;1-methoxy-4-(methanesulfonyl)benzene;methyl-(4-methoxyphenyl)-sulfone;1-methoxy-4-methylsulfonylbenzene
CAS
3517-90-6
化学式
C8H10O3S
mdl
MFCD00025069
分子量
186.232
InChiKey
KAZUCVUGWMQGMC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:120-121.5°C
计算性质
-
辛醇/水分配系数(LogP):0.8
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:51.8
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2909309090
-
危险性防范说明:P233,P260,P261,P264,P270,P271,P280,P301+P312,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P322,P330,P332+P313,P337+P313,P340,P362,P363,P403,P403+P233,P405,P501
-
危险性描述:H302,H312,H315,H319,H332,H335
-
储存条件:室温条件下。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Methylsulfonylanisole
Synonyms: 1-Methoxy-4-(methylsulfonyl)benzene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Methylsulfonylanisole
CAS number: 3517-90-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H10O3S
Molecular weight: 186.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Methylsulfonylanisole
Synonyms: 1-Methoxy-4-(methylsulfonyl)benzene
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Methylsulfonylanisole
CAS number: 3517-90-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H10O3S
Molecular weight: 186.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methoxy-4-(methylsulfinyl)benzene 3517-99-5 C8H10O2S 170.232 4-甲氧基茴香硫醚 1-methoxy-4-methylsulfanyl-benzene 1879-16-9 C8H10OS 154.233 苯甲砜 Methyl phenyl sulfone 3112-85-4 C7H8O2S 156.205 —— 2-((4-methoxyphenyl)sulfinyl)acetic acid 3996-45-0 C9H10O4S 214.242 —— 1,4-bis(methylsulfonyl)benzene 22821-86-9 C8H10O4S2 234.297 对甲氧基苯磺酰胺 4-methoxybenzene sulfonamide 1129-26-6 C7H9NO3S 187.219 对甲氧基苯磺酰氯 4-methoxy-phenyl-sulphonyl chloride 98-68-0 C7H7ClO3S 206.65 4-溴苯甲砜 4-bromoohenyl methyl sulfone 3466-32-8 C7H7BrO2S 235.101 1-碘-4-甲砜基苯 1-iodo-4-(methylsulfonyl)benzene 64984-08-3 C7H7IO2S 282.102 对甲砜基甲苯 Methyl p-tolyl sulfone 3185-99-7 C8H10O2S 170.232 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (S)-p-methoxyphenyl methyl sulfoxide —— C8H10O2S 170.232 —— (R)-4-methoxyphenyl methyl sulfoxide 3517-99-5 C8H10O2S 170.232 4-甲基磺酰苯酚 4-(methylsulfonyl)phenol 14763-60-1 C7H8O3S 172.205 4-甲氧基茴香硫醚 1-methoxy-4-methylsulfanyl-benzene 1879-16-9 C8H10OS 154.233 —— 1-methoxy-4-(phenethylsulfonyl)benzene 203247-70-5 C15H16O3S 276.356 —— 1-(benzylsulfonyl)-4-methoxybenzene 14223-10-0 C14H14O3S 262.329 对甲氧基苯磺酰胺 4-methoxybenzene sulfonamide 1129-26-6 C7H9NO3S 187.219 1-甲氧基-4-((4-甲基苄基)磺酰基)苯 1-methoxy-4-((4-methylbenzyl)sulfonyl)benzene 108545-54-6 C15H16O3S 276.356 对甲砜基甲苯 Methyl p-tolyl sulfone 3185-99-7 C8H10O2S 170.232
反应信息
-
作为反应物:描述:参考文献:名称:WO2006/47302摘要:公开号:
-
作为产物:描述:参考文献:名称:从苯胺到芳醚:一种温和条件下的简便,高效且多功能的合成方法摘要:我们通过醇/酚(ROH)与芳基铵盐(ArNMe反应开发了用于芳基醚的合成的简单且直接的方法3 +),它很容易从苯胺制备(ArNR' 2,R'= H或Me) 。该反应在室温下顺利地且快速地进行(在几个小时内)在市售的碱,例如KO的存在吨卜或KHMDS,并具有相对于二者ROH和ArNR'宽的底物范围2。它具有可扩展性,并且与各种功能组兼容。DOI:10.1002/anie.201712618
文献信息
-
Controlled α-mono- and α,α-di-halogenation of alkyl sulfones using reagent–solvent halogen bonding作者:Christopher M. Poteat、Vincent N. G. LindsayDOI:10.1039/c9cc00550a日期:——The direct and selective α-mono-bromination of alkyl sulfones was achieved through base-mediated electrophilic halogenation. The appropriate combination of solvent and electrophilic bromine source was found to be critical to control the nature of the products formed, where reagent–solvent halogen bonding is proposed to control the selectivity via alteration of the effective size of the electrophilic
-
Polyoxomolybdate-Calix[4]arene Hybrid: A Catalyst for Sulfoxidation Reactions with Hydrogen Peroxide作者:Sara Meninno、Alessandro Parrella、Giovanna Brancatelli、Silvano Geremia、Carmine Gaeta、Carmen Talotta、Placido Neri、Alessandra LattanziDOI:10.1021/acs.orglett.5b02607日期:2015.10.16polyoxomolybdate–calix[4]arene hybrid 1 has been synthesized and applied as a heterogeneous catalyst in the sulfoxidation of thioethers to sulfoxides and to sulfones under strictly stoichiometric amounts of 30% H2O2 in CH3CN as the solvent. This study represents the first promising example of successful employment of calixarenes–polyoxometalate (POM) hybrid materials in the area of catalytic oxidations.
-
A Chemoselective Oxidation of Sulfides to Sulfoxides and Sulfones Using Urea-2,2-dihydroperoxypropane as a Novel Oxidant作者:Kaveh Khosravi、Shirin Naserifar、Atefeh AsgariDOI:10.2174/1570178614666161123115100日期:2017.1.3they have been utilized in several oxidation processes. Methods: We carried out a chemoselective oxidation of sulfides to sulfoxides and sulfones on treatment with urea-2,2-dihydroperoxypropane, a solid oxidant composed of equal amounts of 2,2- dihydroperoxypropane and urea, using THF as the solvent under catalyst-free conditions at room temperature. Results: Sulfides possessing a variety of substitutions背景:亚砜和砜因其在各种方法中的广泛应用而备受关注。这些化合物中存在的官能团是许多天然,药物和农业化合物中的重要组成部分。这些指示物是通过许多路线准备的,并伴随着一些缺点。因此,人们越来越感兴趣的是寻找一种新的方法来通过环境友好的途径生产高产率的这些化合物。近来,宝石二氢过氧化物由于其氧化能力而备受关注,并已用于多种氧化过程中。 方法:我们在无催化剂的情况下,以THF为溶剂,在脲-2,2-二氢过氧丙烷(一种由等量的2,2-二氢过氧丙烷和尿素组成的固体氧化剂)处理下,将硫化物化学选择性氧化为亚砜和砜。在室温条件下。 结果:对具有多种取代基的硫化物(即二烷基,二芳基,烯丙基和烷基芳基)进行了优化的反应条件,根据所用氧化剂的量,它们可以成功地提供不同量的亚砜和砜。根据结果,给电子基团加速了反应,而吸电子取代基降低了反应性。 结论:脲-2,2-二氢过氧丙烷作为固体氧化剂可保存数月而活性没有损失
-
Highly atom-economic, catalyst- and solvent-free oxidation of sulfides into sulfones using 30% aqueous H2O2作者:Marjan JerebDOI:10.1039/c2gc36073j日期:——Highly atom-efficient oxidation of sulfides into sulfones under solvent- and catalyst-free reaction conditions using a 30% aqueous solution of H2O2 at 75 °C is reported. A structurally diverse set of phenyl alkyl-, phenyl benzyl-, benzyl alkyl-, dialkyl-, heteroaryl alkyl- and cyclic sulfides were transformed into sulfones regardless of the aggregate state and electronic nature of the substituents原子效率高 氧化作用 的 硫化物 进入 砜类 在下面 溶剂- 和 催化剂报道了在75℃下使用30%的H 2 O 2水溶液的无反应条件。结构上多样化的一组苯基 烷基-, 苯基 苄基-, 苄基 烷基-,二烷基-, 杂芳基 烷基-和循环 硫化物 被转化为 砜类与取代基的聚集状态和电子性质无关。尽管整个工作过程中反应混合物均不均匀,但没有发现搅拌困难和反应进展的问题。在许多情况下,仅使用过量10 mol%的H 2 O 2,因此大大提高了该方法的高原子经济性。一些固体基材需要可变过量的过氧化氢; 但是,反应是严格进行的,没有有机物溶剂。事实证明,这种转变适合液体和固体放大硫化物。此外,隔离和纯化 的原油产品可以仅用 过滤 和 结晶。
-
[EN] COMPOSITES, METHODS AND USES THEREOF<br/>[FR] COMPOSITES, PROCÉDÉS ET UTILISATIONS ASSOCIÉS申请人:NAT UNIV SINGAPORE公开号:WO2021107872A1公开(公告)日:2021-06-03The present invention relates, in general terms, to methods of catalysing a reaction, including the steps of contacting a chemical entity comprising a sulphide moiety with a composite and an oxidant. The composite acts as a heterogeneous catalyst to oxidise the sulphide moiety. The present invention also relates to composites, methods of synthesising the composites and its use as a catalyst thereof.
表征谱图
-
氢谱1HNMR
-
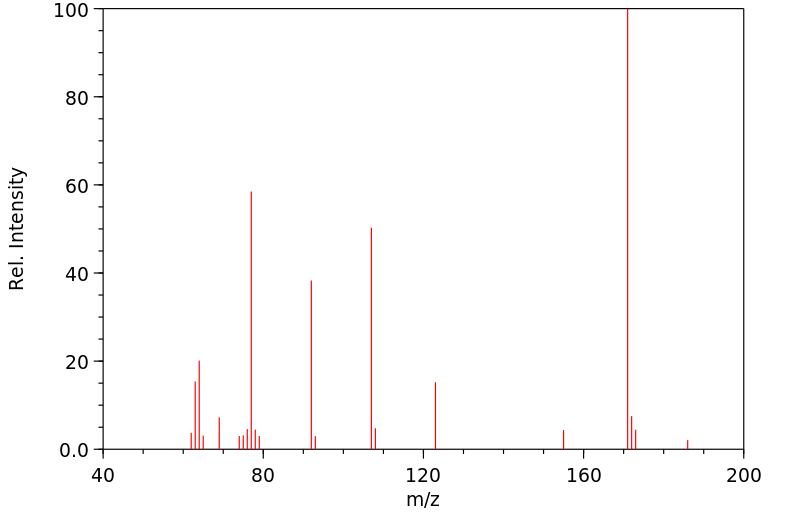
质谱MS
-
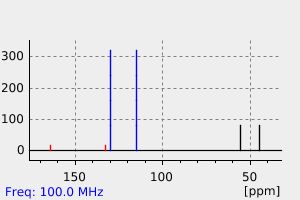
碳谱13CNMR
-
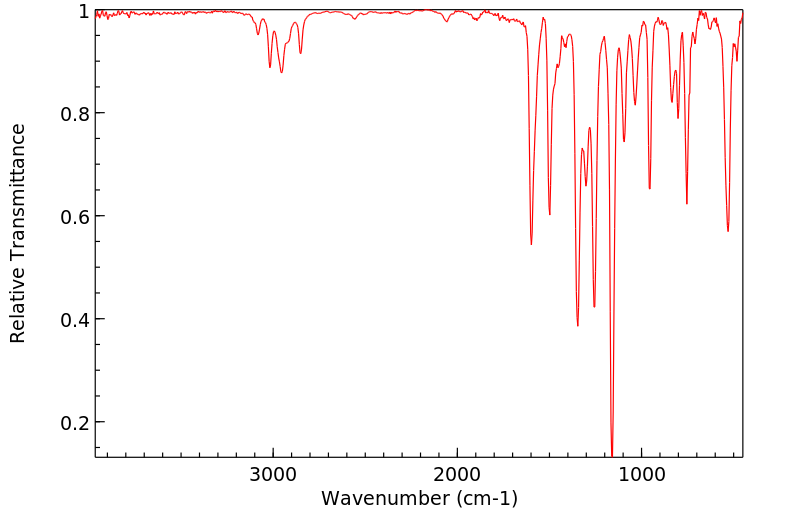
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫