戊-3-炔-2-酮 | 7299-55-0
中文名称
戊-3-炔-2-酮
中文别名
——
英文名称
pent-3-yn-2-one
英文别名
3-pentyn-2-one;2-Pentyn-4-one
CAS
7299-55-0
化学式
C5H6O
mdl
——
分子量
82.102
InChiKey
DZOOXMGZVWHNAS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:6
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
储存条件:存储条件:2-8°C,避光,惰性气体
SDS
反应信息
-
作为反应物:参考文献:名称:Yvon, Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1925, vol. 180, p. 749摘要:DOI:
-
作为产物:参考文献:名称:Petrow; Porfir'ewa, Doklady Akademii Nauk SSSR, 1953, vol. 90, p. 561,562,563摘要:DOI:
文献信息
-
Total Synthesis of Hematoporphyrin and Protoporphyrin: A Conceptually New Approach作者:Pierre Martin、Markus Mueller、Dietmar Flubacher、Andreas Boudier、Hans-Ulrich Blaser、Dirk SpielvogelDOI:10.1021/op100036c日期:2010.7.16The total synthesis of protoporphyrin IX and its disodium salt using a new alternative method to the classical MacDonald condensation is reported. The key step is the reaction of the new unsymmetrical diiodo dipyrrylmethane 1 with the known dipyrrylmethane 2. Coupling of the two fragments leads directly to porphyrin 3 without the need of an oxidizing agent. The new methodology is well suited for the
-
Selective Alkenylation and Hydroalkenylation of Enol Phosphates through Direct CH Functionalization作者:Xu‐Hong Hu、Xiao‐Fei Yang、Teck‐Peng LohDOI:10.1002/anie.201506437日期:2015.12.14Rh‐catalyzed direct CH functionalization reaction of enol phosphates was developed. The method is applicable to a variety of coupling partners, including activated alkenes, alkynes, and allenes, and leads to the formation of various valuable alkenylated and hydroalkenylated enol phosphates through the action of the phosphate directing group. The versatility and utility of the coupling products were demonstrated
-
Palladium-Catalyzed Additions of Terminal Alkynes to Acceptor Alkynes作者:Barry M. Trost、Mark T. Sorum、Chuen Chan、Gerd RühterDOI:10.1021/ja9624937日期:1997.1.1The development of addition reactions wherein the product is the simple sum of the reactants plus anything else (only needed catalytically) constitutes an important goal for enhanced synthetic efficiency. The C−H bond of terminal alkynes (the donor alkynes) can be added to either terminal alkynes (self-coupling) or activated internal alkynes (cross-coupling) (the acceptor alkynes) in the presence of
-
Process For Preparing Porphyrin Derivatives, Such As Protoporphyrin (IX) And Synthesis Intermediates申请人:Martin Pierre公开号:US20080242857A1公开(公告)日:2008-10-02The present invention relates to a process for preparing a porphyrin of formula (I), optionally in the form of a salt with an alkali metal and/or in the form of a metal complex: in which: R and R′ are as defined in claim 1 , comprising: a step of condensation, in an acidic medium, between a dipyrromethane of formula (II): in which R′b is as defined above for (I), and a dipyrromethane of formula (III): in which R″ is as defined in claim 1 , and also the compounds of formula (III).
-
Stereoselective Synthesis of the C1–C22 Carbon Framework of (−)-Amphidinolide K作者:Somnath S. Chandankar、Sadagopan RaghavanDOI:10.1021/acs.joc.9b01248日期:2019.8.2Two stereoselective routes to the C7–C22 subunit of amphidinolide K are disclosed. Jacobsen’s hydrolytic kinetic resolution and Sharpless’ asymmetric dihydroxylation reactions have been employed for the construction of the tetrahydrofuran ring. The C10–C11, C16–C17, C9–O, and C18–O bonds have been created using α-chloro sulfide intermediates and [2,3] sigmatropic rearrangement. Marshall’s propargylation
表征谱图
-
氢谱1HNMR
-
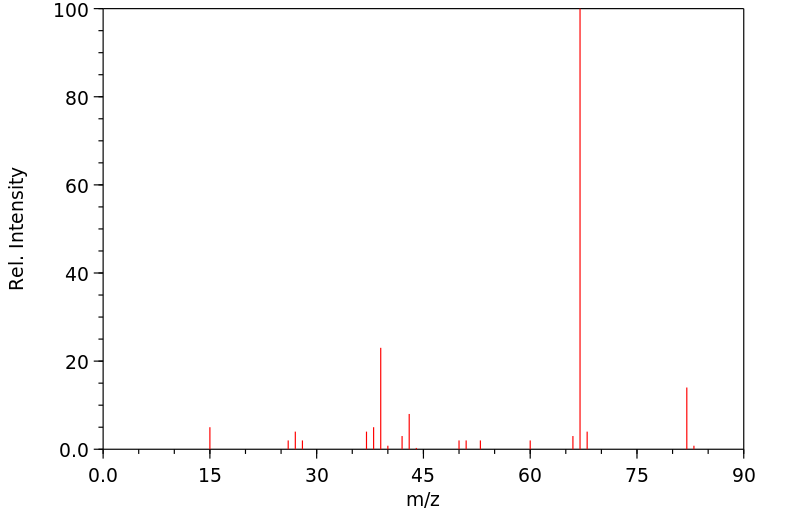
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷