4-甲基-1-庚烯-4-醇 | 1186-31-8
中文名称
4-甲基-1-庚烯-4-醇
中文别名
4-甲-1-庚烯-4-醇;烯丙基甲基丙基甲醇
英文名称
4-methylhept-1-en-4-ol
英文别名
4-Methyl-hepten-(1)-ol-(4);4-Methyl-1-hepten-4-ol
CAS
1186-31-8
化学式
C8H16O
mdl
——
分子量
128.214
InChiKey
PYKQMQBNPLNLPS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-31.5°C (estimate)
-
沸点:202.67°C (estimate)
-
密度:0,83 g/cm3
-
闪点:51°(124°F)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:3
-
海关编码:2905290000
-
危险类别:3
SDS
反应信息
-
作为反应物:描述:4-甲基-1-庚烯-4-醇 在 potassium tert-butylate 、 双氧水 、 对硝基苯磺酰氯 、 lithium diisopropyl amide 作用下, 以 四氢呋喃 、 二氯甲烷 为溶剂, 反应 42.5h, 生成参考文献:名称:一种2-羟甲基氧杂环丁烷衍生物的制备方法 和应用摘要:本发明公开了一种2‑羟甲基氧杂环丁烷衍生物的制备方法和应用,包括以下步骤:以化合物II和化合物III为原料,发生巴比耶反应得到化合物IV;化合物IV中的烯键成环氧后得到化合物V;在碱性条件下开环生成化合物VI;经过关环反应后得到化合物VII,最后脱苄基保护基得到2‑羟甲基氧杂环丁烷衍生物(化合物I)。公开号:CN108863991B
-
作为产物:描述:4-methyl-1-tetrahydropyran-2-yloxy-hept-2-yn-4-ol 生成 4-甲基-1-庚烯-4-醇参考文献:名称:A Novel Stereoselective Synthesis oftransHomoallylic Alcohols from Acetylenes1摘要:DOI:10.1055/s-1973-22255
文献信息
-
Allylation of Unactivated Ketones by Tetraallyltin Accelerated by Phenol. Application to Asymmetric Allylation Using a Tetraallyltin-BINOL System作者:Makoto Yasuda、Noriko Kitahara、Tatsuya Fujibayashi、Akio BabaDOI:10.1246/cl.1998.743日期:1998.8The tetraallyltin-phenol system was mild and effective for allylation of unactivated ketones, giving tertiary alcohols in high yields. The asymmetric allylation was achieved by a tetraallyltin-homochiral BINOL (1,1′-bi-2-naphthol) system. The addition of methanol raised the enantioselectivity to afford the tertiary homoallylic alcohol up to 60% ee.
-
ALDEHYDE-SELECTIVE WACKER-TYPE OXIDATION OF UNBIASED ALKENES申请人:CALIFORNIA INSTITUTE OF TECHNOLOGY公开号:US20140316149A1公开(公告)日:2014-10-23This disclosure is directed to methods of preparing organic aldehydes, each method comprising contacting a terminal olefin with an oxidizing mixture comprising: (a) a dichloro-palladium complex; (b) a copper complex; (c) a source of nitrite; under aerobic reaction conditions sufficient to convert at least a portion of the terminal olefin to an aldehyde.
-
SnCl2-mediated carbonyl allylation of aldehydes and ketones in ionic liquid作者:Long Tang、Li Ding、Wei-Xing Chang、Jing LiDOI:10.1016/j.tetlet.2005.11.022日期:2006.1In ionic liquid [bmim]BF4, SnCl2·2H2O acts as an inexpensive and efficient metal salt for carbonyl allylation. By applying ionic liquid, some previously reported serious operational problems associated with the SnCl2-mediated allylation reaction are avoided. Furthermore, ketones, which are less reactive than aldehydes, can also be allylated in high yields with this system.
-
Catalyst-Controlled Wacker-Type Oxidation: Facile Access to Functionalized Aldehydes作者:Zachary K. Wickens、Kacper Skakuj、Bill Morandi、Robert H. GrubbsDOI:10.1021/ja411749k日期:2014.1.22The aldehyde-selective oxidation of alkenes bearing diverse oxygen groups in the allylic and homoallylic position was accomplished with a nitrite-modified Wacker oxidation. Readily available oxygenated alkenes were oxidized in up to 88% aldehyde yield and as high as 97% aldehyde selectivity. The aldehyde-selective oxidation enabled the rapid, enantioselective synthesis of an important pharmaceutical
-
COPPER (I)-CATALYZED REACTION OF ISOPRENE BROMOHYDRIN WITH ORGANOLITHIUM REAGENT作者:Shuki Araki、Yasuo ButsuganDOI:10.1246/cl.1980.185日期:1980.2.5The reaction of isoprene bromohydrin (1-bromo-2-methyl-3-buten-2-ol) (1) with ethyl-, propyl- and butyllithium in the presence of copper (I) iodide leads to the exclusive formation of the vinyl group migrated 2-alkyl-4-penten-2-ols (3d–3f), in contrast to the reaction with methyl-, phenyl- and benzyllithium, which yields the corresponding 2-methyl-4-substituted-2-buten-l-ols (2a–2c).
表征谱图
-
氢谱1HNMR
-
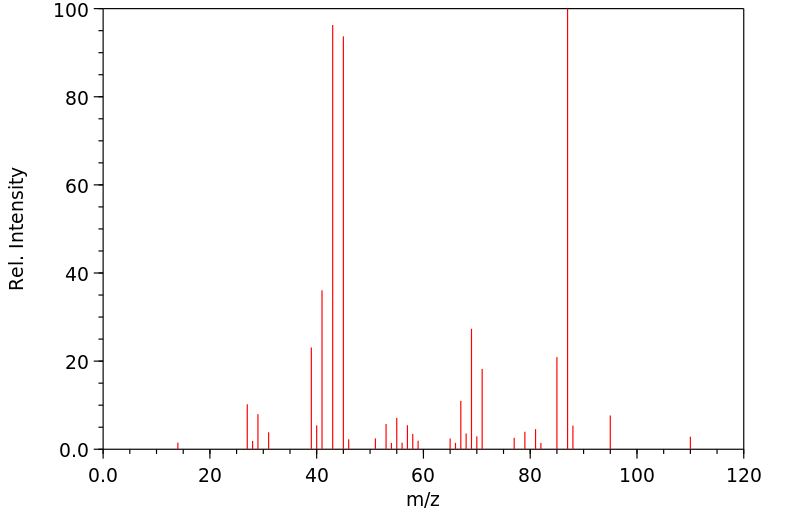
质谱MS
-
碳谱13CNMR
-
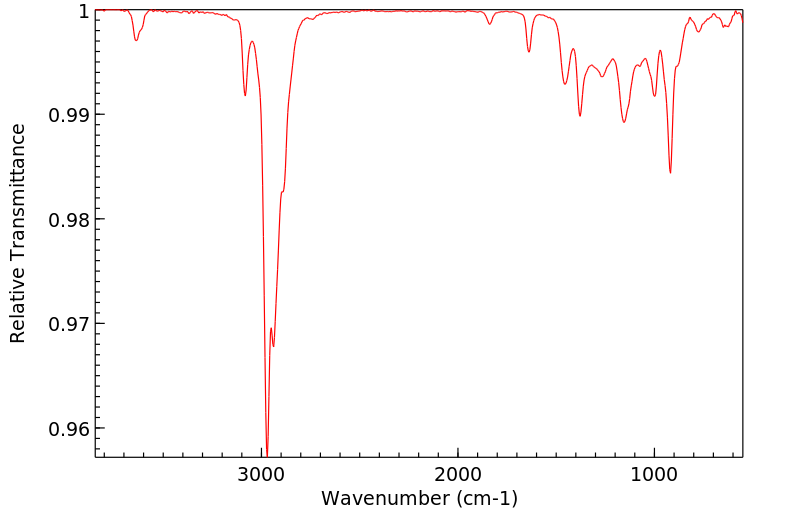
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷