3-(4-吗啉基)苯酚 | 27292-49-5
中文名称
3-(4-吗啉基)苯酚
中文别名
3-吗啉苯酚
英文名称
3-(4-morpholino)phenol
英文别名
3-morpholinophenol;N-m-hydroxyphenylmorpholine;m-Morpholino-phenol;3-(4-morpholinyl)-phenol;AMA58;3-morpholin-4-ylphenol
CAS
27292-49-5
化学式
C10H13NO2
mdl
MFCD00051675
分子量
179.219
InChiKey
BMGSGGYIUOQZBZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:136 °C
-
沸点:362.6±37.0 °C(Predicted)
-
密度:1.185±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:32.7
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
WGK Germany:3
-
危险性防范说明:P233,P260,P261,P264,P270,P271,P280,P301+P312,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P330,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:保持贮藏器密封,并将其放入一个紧密的容器中。存放在阴凉、干燥的地方。
SDS
| Name: | 3-Morpholinophenol 97% Material Safety Data Sheet |
| Synonym: | N-(3-Hydroxyphenyl)morpholin |
| CAS: | 27292-49-5 |
Synonym:N-(3-Hydroxyphenyl)morpholin
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 27292-49-5 | 3-Morpholinophenol | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 27292-49-5: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: pink
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 132 - 134 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H13NO2
Molecular Weight: 179.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents, acids, bases, acid chlorides.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 27292-49-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Morpholinophenol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 27292-49-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 27292-49-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 27292-49-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-(3-苯基甲氧基苯基)吗啉 4-(3-benzyloxy-phenyl)-morpholine 26926-56-7 C17H19NO2 269.343 3-(4-吗啉基)苯胺 3-morpholine-4-ylaniline 159724-40-0 C10H14N2O 178.234 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-amino-5-morpholinophenol 25912-15-6 C10H14N2O2 194.233 4-(3-甲氧基苯基)吗啉 4-(3-methoxyphenyl)morpholine 32040-09-8 C11H15NO2 193.246 —— 4-(3-(allyloxy)phenyl)morpholine 1620293-44-8 C13H17NO2 219.283 —— 5-Morpholino-2-nitrosophenol 27292-55-3 C10H12N2O3 208.217 2-羟基-4-N-吗啉基苯甲醛 2-hydroxy-4-morpholinobenzaldehyde 70362-07-1 C11H13NO3 207.229 —— N-<3-(2,3-Epoxypropoxy)phenyl>morpholin —— C13H17NO3 235.283 —— 5,5'-bis-morpholino-2,2'-methandiyl-di-phenol 1229439-21-7 C21H26N2O4 370.448 1-(2-羟基-4-N-吗啉基苯基)乙酮 IC86621 404009-40-1 C12H15NO3 221.256 —— 1-morpholin-4-yl-3-phenylcarbamoyloxy-benzene 37895-05-9 C17H18N2O3 298.342 —— 2-hydroxy-4-morpholinobenzamide 37893-38-2 C11H14N2O3 222.244 —— 4-(3-fluorophenyl)morpholine —— C10H12FNO 181.21 —— 3-morpholin-4-ylphenyl trifluoromethanesulfonate 597584-59-3 C11H12F3NO4S 311.282 —— 2-chloro-1-(2-hydroxy-4-morpholin-4-yl-phenyl)-ethanone 404010-44-2 C12H14ClNO3 255.701 DNA-PK抑制剂V 1-(2-hydroxy-4-morpholin-4-ylphenyl)phenylmethanone 404009-46-7 C17H17NO3 283.327 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Isoform-specific phosphoinositide 3-kinase inhibitors from an arylmorpholine scaffold摘要:Phosphoinositide 3-kinases (PI3-Ks) are an ubiquitous class of signaling enzymes that regulate diverse cellular processes including growth, differentiation, and motility. Physiological roles of PI3-Ks have traditionally been assigned using two pharmacological inhibitors, LY294002 and wortmannin. Although these compounds are broadly specific for the PI3-K family, they show little selectivity among family members, and the development of isoform-specific inhibitors of these enzymes has been long anticipated. Herein, we prepare compounds from two classes of arylmorpholine PI3-K inhibitors and characterize their specificity against a comprehensive panel of targets within the PI3-K family. We identify multiplex inhibitors that potently inhibit distinct subsets of PI3-K isoforms, including the first selective inhibitor of p110beta/p110delta (IC50 p110beta = 0.13 muM, p110delta = 0.63 muM). We also identify trends that suggest certain PI3-K isoforms may be more sensitive to potent inhibition by arylmorpholines, thereby guiding future drug design based on this pharmacophore. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2004.06.022
-
作为产物:描述:3-苄氧基溴苯 在 tris-(dibenzylideneacetone)dipalladium(0) 、 palladium 10% on activated carbon 氢气 、 R-(+)-1,1'-联萘-2,2'-双二苯膦 、 sodium t-butanolate 作用下, 以 甲醇 、 乙酸乙酯 、 甲苯 为溶剂, 80.0 ℃ 、275.8 kPa 条件下, 反应 56.0h, 生成 3-(4-吗啉基)苯酚参考文献:名称:[EN] PHARMACEUTICAL COMPOSITION FOR PREVENTING AND TREATING METABOLIC BONE DISEASES CONTAINING ALPHA-ARYLMETHOXYACRYLATE DERIVATIVES
[FR] COMPOSITION PHARMACEUTIQUE DESTINEE A PREVENIR ET A TRAITER DES MALADIES OSSEUSES METABOLIQUES, CONTENANT DES DERIVES D'ALPHA-ARYLMETHOXYACRYLATE摘要:本发明涉及使用特定的α-芳基甲氧基丙烯酸酯衍生物,或其药理学上可接受的盐或溶剂,用于预防和治疗代谢性骨疾病。公开号:WO2005123054A1
文献信息
-
[DE] 2-HETEROARYLCARBONSÄUREAMIDE<br/>[EN] 2-HETEROARYL CARBOXAMIDES<br/>[FR] 2-HETEROARYLCARBOXAMIDES申请人:BAYER HEALTHCARE AG公开号:WO2003104227A1公开(公告)日:2003-12-18Die Erfindung betrifft neue 2-Heteroarylcarbonsäureamide und ihre Verwendung zur Herstellung von Arzneimitteln zur Behandlung und/oder Prophylaxe von Krankheiten und zur Verbesserung der Wahrnehmung, Konzentrationsleistung, Lernleistung und/oder Gedächtnisleistung. (I): in welcher R1 1-Aza-bicyclo [2.2.2]oct-3-yl, welches gegebenenfalls über das Sticktoffätom mit einem Rest ausgewählt aus der Gruppe C1-C4-Alkyl, Benzyl und Oxy substituiert ist, A Sauerstoff oder Schwefel, der Ring B Benzo oder Pyrido, die jeweils gegebenenfalls durch Reste aus der Reihe Halogen, Cyano, Formyl, Trifluormethyl, Trifluormethoxy, Nitro, Amino, C1-C6-Alkyl und C1-C6-Alkoxy substituiert sind, E C≡C, Aryl und Heteroaryl, wobei Aryl und Heteroaryl durch Reste aus der Reihe Halogen, Cyano, Trifluormethyl, Trifluormethyl, Trifluormethoxy, Nitro, Amino, C1-C6-Alkoxy und C1-C6-Alkyl substituiert sein Können, bedeuten, sowie die Solvate, Salze oder Solvate der Salze dieser Verbindungen.这项发明涉及新的2-杂环芳基羧酰胺及其用于制备用于治疗和/或预防疾病以及改善感知、注意力、学习和/或记忆能力的药物的用途。其中,R1为1-Aza-bicyclo[2.2.2]oct-3-yl,可能通过氮原子与来自C1-C4-烷基、苄基和氧的基团中的一种取代,A为氧或硫,环B为苯并或吡啶,并且可以通过来自卤素、氰基、甲酰基、三氟甲基、三氟甲氧基、硝基、氨基、C1-C6-烷基和C1-C6-烷氧基的基团取代,E为C≡C、芳基和杂环芳基,其中芳基和杂环芳基可以通过来自卤素、氰基、三氟甲基、三氟甲基、三氟甲氧基、硝基、氨基、C1-C6-烷氧基和C1-C6-烷基的基团取代,以及这些化合物的溶剂化合物、盐或盐的溶剂化合物。
-
A Novel Morpholine-Based Rhodamine Fluorescent Chemosensor for the Rapid Detection of Hg<sup>2+</sup> Ions作者:Hwalkee Park、Mergu Naveen、Kyoung-Lyong An、Kun Jun、Young-A SonDOI:10.1166/jnn.2019.16709日期:2019.11.1
A novel rhodamine-based receptor bearing a morpholine (RDM) was developed as a fluorescent chemosensor with high selectivity toward Hg2+. After the addition of Hg2+ to RDM, the color of the solution changed from colorless to pink, and the new absorption band appears at 580 nm. The fluorescent of RDM appears to orange color in the presence of Hg2+. Upon the addition of Hg2+, ring-opening of the corresponding spirolactam gives rise to fluorescence, and a 1:1 metal-ligand complex formed.
-
[EN] 3-ARYLOXY AND 3-HETEROARYLOXY SUBSTITUTED BENZO(B) THIOPHENES AS THERAPEUTIC AGENTS WITH PI3K ACTIVITY<br/>[FR] BENZO[B]THIOPHENES A SUBSTITUTION 3-ARYLOXY ET 3-HETEROARYLOXY EN TANT QU'AGENTS THERAPEUTIQUES A ACTIVITE PI3K申请人:WARNER LAMBERT CO公开号:WO2004108715A1公开(公告)日:2004-12-16The present invention provides benzo[b]thiophenes of Formula (I), wherein R1, R2, R3, R4, R5, and L have any of the values defined therefor in the specification, and pharmaceutically acceptable salts thereof, that are useful as agents in the treatment of diseases and conditions, including inflammatory diseases, cardiovascular diseases, and cancers. Also provided are pharmaceutical compositions comprising one or more compounds of Formula (I).本发明提供了式(I)的苯并[b]噻吩,其中R1、R2、R3、R4、R5和L具有规范中为其定义的任何值,以及药用可接受的盐,这些盐作为治疗疾病和状况的药剂是有用的,包括炎性疾病、心血管疾病和癌症。另外还提供了包含一个或多个式(I)化合物的药物组合物。
-
[EN] PROCESS FOR DEOXYFLUORINATION OF PHENOLS<br/>[FR] PROCÉDÉ DE DÉSOXYFLUORATION DE PHÉNOLS申请人:STUDIENGESELLSCHAFT KOHLE MBH公开号:WO2019025137A1公开(公告)日:2019-02-07The present invention refers to a process for transition-meta!-assisted 18F- deoxyfluorination of phenols. The transformation benefits from readily available phenols as starting materials; tolerance of moisture and ambient atmosphere, large substrate scope, and translatability to generate doses appropriate for positron emission tomography (PET) imaging.
-
Novel amino acid ester and reagent composition for detecting leucocytes or elastase in body liquid申请人:KYOTO DAIICHI KAGAKU CO., LTD.公开号:EP0661280A1公开(公告)日:1995-07-05An amino acid ester of the formula: (X-O-A-)n-Y in which X is derived from an aromatic or heterocyclic compound: X-OH or its derivative; A is a residue of a L-amino acid, n is an integer of at least 2, and Y is a N-substituent of an amino acid derived from a compound having the same or different two or more carbonyl or sulfonyl groups which is formed from a single or complex compound selected from the group consisting of noncyclic or cyclic hydrocarbons having 1 to 10 carbon atoms, saturated or unsaturated heterocyclic compounds, aromatic compounds, organosilicon compounds having 1 to 10 silicon atoms, monosaccharides and oligosaccharide comprising 2 to 10 monosaccharides, all of which may have a substituent and/or a hetero atom to form the complex, which ester is specifically hydrolyzed by leucocytes or elastase.
表征谱图
-
氢谱1HNMR
-
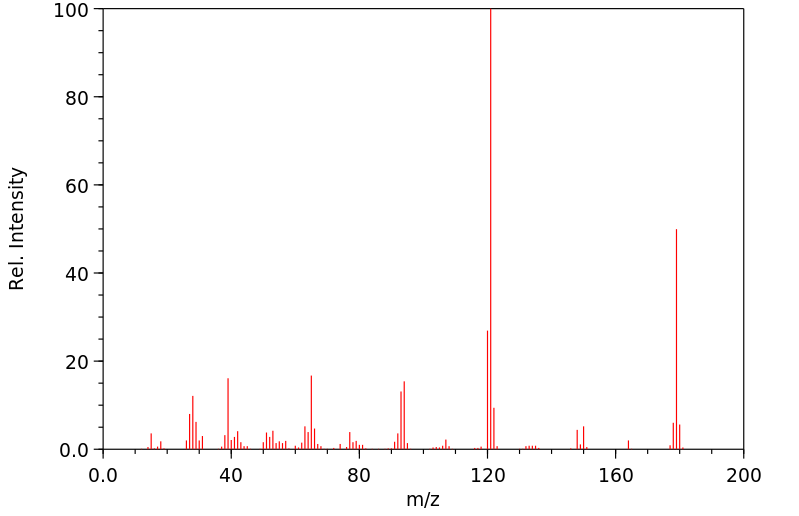
质谱MS
-
碳谱13CNMR
-
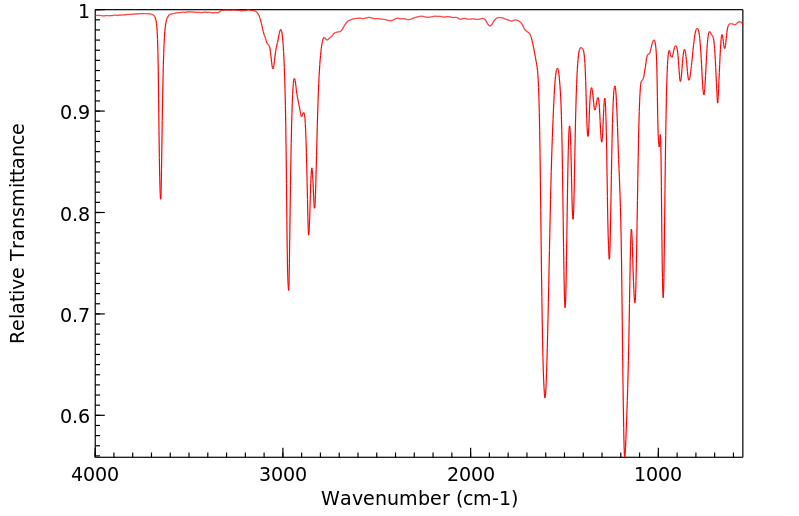
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(4-甲基环戊-1-烯-1-基)(吗啉-4-基)甲酮
(2-肟基-氰基乙酸乙酯)-N,N-二甲基-吗啉基脲六氟磷酸酯
鲸蜡基乙基吗啉氮鎓乙基硫酸盐
马啉乙磺酸钾
预分散OTOS-80
顺式4-(氮杂环丁烷-3-基)-2,2-二甲基吗啉
顺式-N-亚硝基-2,6-二甲基吗啉
顺式-3,5-二甲基吗啉
顺-2,6-二甲基-4-(4-硝基苯基)吗啉
非屈酯
雷奈佐利二聚体
阿瑞杂质9
阿瑞杂质12
阿瑞吡坦磷的二卞酯
阿瑞吡坦杂质
阿瑞吡坦杂质
阿瑞吡坦EP杂质C
阿瑞吡坦
阿瑞吡坦
阿瑞匹坦非对映异构体2R3R1R
阿瑞匹坦杂质A异构体
阿瑞匹坦杂质54
阿瑞匹坦-M3代谢物
钾[2 - (吗啉- 4 -基)乙氧基]甲基三氟硼酸
酮康唑杂质
邻苯二甲酸单吗啉
调节安
试剂2-(4-Morpholino)ethyl2-bromoisobutyrate
茂硫磷
苯甲腈,2-(4-吗啉基)-5-[1,4,5,6-四氢-4-(羟甲基)-6-羰基-3-哒嗪基]-
苯甲曲秦
苯甲吗啉酮
苯基2-(2-苯基吗啉-4-基)乙基碳酸酯盐酸盐
苯二甲吗啉一氢酒石酸盐
苯二甲吗啉
苯乙酮 O-(吗啉基羰基甲基)肟
芬美曲秦
芬布酯盐酸盐
芬布酯
脾脏酪氨酸激酶(SYK)抑制剂
脱氯利伐沙班
脱氟雷奈佐利
羟基1-(3-氯苯基)-2-[(1,1-二甲基乙基)氨基]-1-丙酮盐酸盐
福沙匹坦苄酯
福沙匹坦杂质26
福沙匹坦N-苄基杂质
福曲他明
碘化N-甲基丙基吗啉
碘化N-甲基,乙基吗啉
硝酸吗啉