三甲基(1-(三甲基硅烷基)乙烯基)硅烷 | 5654-07-9
中文名称
三甲基(1-(三甲基硅烷基)乙烯基)硅烷
中文别名
——
英文名称
1,1-bis(trimethylsilyl)ethylene
英文别名
1,1-bis(trimethylsilyl)ethene;trimethyl(1-trimethylsilylethenyl)silane
CAS
5654-07-9
化学式
C8H20Si2
mdl
——
分子量
172.418
InChiKey
CVCQWLSMIOKNLE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:150-151 °C
-
密度:0.7929 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:10
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Groebel,B.-T.; Seebach,D., Chemische Berichte, 1977, vol. 110, p. 867 - 877摘要:DOI:
-
作为产物:描述:参考文献:名称:Synthesis of Bis(trimethylsilyl) Ketone and Reactions with Organometallic Compounds.摘要:Bis(trimethylsilyl) ketone (1) has been prepared by hydrolysis of the cr-chloro ether 3a on silica gel. Reaction of ketone 1 with organoaluminium, organomagnesium and organolithium compounds gave addition products and/or bis(trimethylsilyl)methanol(5).DOI:10.3891/acta.chem.scand.52-1141
文献信息
-
10.1021/acs.organomet.0c00517作者:Mendoza-Espinosa, Daniel、Rendón-Nava, David、Vásquez-Pérez, Jose M.、Sandoval-Chávez, Cesar I.、Alvarez-Hernández, AlejandroDOI:10.1021/acs.organomet.0c00517日期:——of complexes 4a–d featuring a [Rh(CO)2I] fragment used for the detemination of the donor properties of the new triazolylidene ligands. All complexes have been fully characterized by means of 1H and 13C NMR spectroscopy, melting point, elemental analysis, and in the case of complex 3a, by X-ray crystallography. Comparison of the catalytic activity of the new rhodium complexes in C–C and C–Si bond forming多齿卡宾配体是制备具有更高核能的卡宾配合物的有价值的框架。在目前的工作中,我们报道了由中离子三唑-5亚基支持的一系列单-至四- [Rh(COD)I]配合物(3a - d)的合成。一般的合成步骤包括在KHMDS和适量的铑(I)前体存在下,适当的三唑鎓(2a - d)盐一步反应。的配合物治疗3A - d与过量的一氧化碳的允许的配合物的制备定量4A - d设有的[Rh(CO)2I]片段用于确定新的三唑基亚配体的供体性质。所有配合物均已通过1 H和13 C NMR光谱,熔点,元素分析以及在配合物3a的情况下通过X射线晶体学进行了充分表征。比较新的铑配合物在C–C和C–Si键形成过程中的催化活性,表明四核物种的性能得到了增强,表明在这些多核配合物中可能产生强力协同作用。
-
A New Facile Synthesis of 1,1-Dibromo-2-arylethenes作者:Bogdan Marciniec、Piotr Pawluć、Grzegorz Hreczycho、Jędrzej WalkowiakDOI:10.1055/s-2007-984901日期:——Synthetically useful 1,1-dibromo-2-arylethenes were readily prepared in good yields via double bromodesilylation of the easily accessible 1,1-bis(trimethylsilyl)-2-arylethenes using N-bromosuccinimide under mild conditions.
-
A New Selective Approach to 1,1-Bis(silyl)-2-arylethenes and 1,1-Bis(silyl)-1,3-butadienes via Sequential Silylative Coupling−Heck Coupling Reactions作者:Piotr Pawluc、Grzegorz Hreczycho、Bogdan MarciniecDOI:10.1021/jo0616254日期:2006.10.1route to 1,1-bis(silyl)-1-alkenes has been developed. Sequential one-pot silylative coupling exo-cyclization of 1,2-bis(dimethylvinylsiloxy)ethane followed by the reaction with Grignard reagents leads to the desired 1,1-bis(silyl)ethenes, which are then efficiently coupled in the presence of silver nitrate and palladium acetate with aryl or alkenyl idodides to give the corresponding 1,1-bis(silyl)-2-arylethenes
-
The reactions of tris(trimethylsilyl)methyl-lithium with some carbon electrophiles作者:Ian Fleming、Christopher D. FloydDOI:10.1039/p19810000969日期:——non-enolisable aldehydes, ketones, and acid chlorides, and with some epoxides, with the formation of carbon–carbon bonds. This method of preparing functionalized silanes is limited by the readiness with which (1) abstracts a proton, if one is available, rather than attacks at carbon. In the reaction with epoxides, the product alkoxide can transfer a silyl group from carbon to oxygen, and in one case the
-
A Facile Synthesis of 1,1-Bis(silyl)ethenes作者:Piotr Pawluc、Bogdan Marciniec、Grzegorz Hreczycho、Beata Gaczewska、Yujiro ItamiDOI:10.1021/jo048784c日期:2005.1.1Symmetrical 1,1-bis(silyl)ethenes have been easily prepared via ruthenium complex-catalyzed silylative coupling cyclization of 1,2-bis(dimethylvinylsiloxy)ethane to give 2,2,4,4-tetramethyl-3-methylene-1,5-dioxa-2,4-disilacycloheptane with excellent selectivity and good yield, followed by its reaction with Grignard reagents. The cyclic product can also be effectively transformed into cyclic carbosiloxane
表征谱图
-
氢谱1HNMR
-
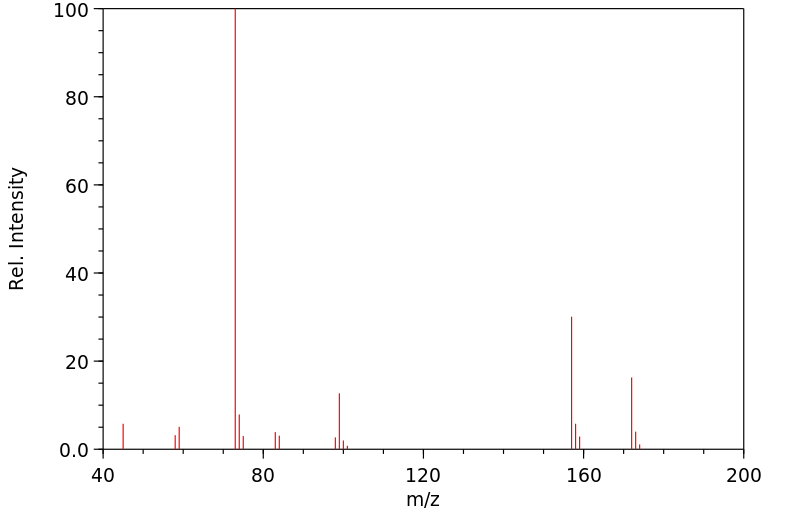
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷