叔壬基硫醇 | 25360-10-5
中文名称
叔壬基硫醇
中文别名
叔壬硫醇;叔九硫醇
英文名称
tert-nonyl mercaptan
英文别名
2-methyloctane-2-thiol;t-nonyl mercaptan;tert-nonanethiol
CAS
25360-10-5
化学式
C9H20S
mdl
MFCD00059963
分子量
160.324
InChiKey
MPBLPZLNKKGCGP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-44.55°C (estimate)
-
沸点:188 °C(lit.)
-
密度:0.856 g/mL at 25 °C(lit.)
-
闪点:104 °F
-
LogP:4.21 at 20℃
-
物理描述:Liquid
-
稳定性/保质期:
避免接触强氧化剂。
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:6.1(b)
-
危险品标志:Xi
-
安全说明:S16,S26,S36
-
危险类别码:R36/37,R10,R43
-
WGK Germany:3
-
危险品运输编号:UN 3336
-
包装等级:III
-
危险类别:6.1(b)
-
储存条件:将物品存放在紧密的容器中,并储存在阴凉、干燥的地方。
反应信息
-
作为反应物:描述:参考文献:名称:REACTIONS OFt-ALKYL THIONITRATES WITHp-AMINOPHENOLS: ONE POT SYNTHESES OFN-(t-ALKYLTHIO)-p-BENZOQUINONEIMINES摘要:多种N-(烷基硫)对苯醌亚胺通过将相应的对氨基酚与叔烷基硫氰酸酯反应简单合成。DOI:10.1246/cl.1979.1077
-
作为产物:参考文献:名称:Structure–Odor Correlations in Homologous Series of Alkanethiols and Attempts To Predict Odor Thresholds by 3D-QSAR Studies摘要:Homologous series of alkane-1-thiols, alkane-2-thiols, alkane-3-thiols, 2-methylalkane-1-thiols, 2-methylalkane-3-thiols, 2-methylalkane-2-thiols, and alkane-1,?-dithiols were synthesized to study the influence of structural changes on odor qualities and odor thresholds. In particular, the odor thresholds were strongly influenced by steric effects: In all homologous series a minimum was observed for thiols with five to seven carbon atoms, whereas increasing the chain length led to an exponential increase in the odor threshold. Tertiary alkanethiols revealed clearly lower odor thresholds than found for primary or secondary thiols, whereas neither a second mercapto group in the molecule nor an additional methyl substitution lowered the threshold. To investigate the impact of the SH group, odor thresholds and odor qualities of thiols were compared to those of the corresponding alcohols and (methylthio)alkanes. Replacement of the SH group by an OH group as well as S-methylation of the thiols significantly increased the odor thresholds. By using comparative molecular field analysis, a 3D quantitative structureactivity relationship model was created, which was able to simulate the odor thresholds of alkanethiols in good agreement with the experimental results. NMR and mass spectrometric data for 46 sulfur-containing compounds are additionally supplied.DOI:10.1021/jf506135c
-
作为试剂:参考文献:名称:单S-脂化肽水凝胶的合成和表征:活性氧响应材料的制备平台摘要:在这项工作中,我们报告了通过光引发硫醇-烯反应( S-脂化)合成在 N 末端含有 3-巯基丙酸酯接头的单脂化肽。我们评估了包含不同长度的脂质链的单S-脂质化肽库的自组装和水凝胶化特性,并证明水凝胶化是由脂质链的疏水性和肽的表面疏水性之间的平衡驱动的。我们进一步假设,在设计类似系统时,使用 log D估计值的简单计算可以用作水凝胶化的预测因子。对含有形成水凝胶的短脂质链的单S-脂化肽进行了充分表征,并开发了肽水凝胶化的机制。最后,我们证明,单S-脂化肽水凝胶中硫醚基团的存在(这是传统N-酰基脂化系统所缺乏的特征)使得能够通过活性氧氧化成亚砜来控制凝胶的分解。根据疏水性调节策略。因此,我们得出结论,单S-脂化肽水凝胶构成了一种新颖且简单的工具,用于开发组织工程和活性氧过度表达疾病(例如退行性和代谢性疾病以及癌症)的靶向药物递送应用。DOI:10.1039/d1ob00355k
文献信息
-
QUENCHER申请人:Wako Pure Chemical Industries, Ltd.公开号:US20170342031A1公开(公告)日:2017-11-30A quencher is disclosed having a compound represented by the following general formula (1): wherein R 5 each independently represent a halogen atom, an alkyl group, an alkoxy group, an alkylthio group, an amino group having a substituent or not having a substituent, a hydroxy group, an aryl group, an aryloxy group, or an arylalkyl group; R 6 represents a group having a polymerizable unsaturated group, a hydroxy group, or the like; Y 1 represents an oxygen atom, or the like; An − represents an anion; Ar 1 represents a specific ring structure; * and ** represent binding positions; Ar 2 represents a benzene ring, a naphthalene ring, or an anthracene ring; n 1 represents a specific integer; and the following structure (1-10) in the general formula (1) is an asymmetric structure; (wherein R 5 , Y 1 , Ar 1 , Ar 2 , n 1 , * and ** are the same as described above.).
-
Photoredox‐Controlled β‐Regioselective Radical Hydroboration of Activated Alkenes with NHC‐Boranes作者:Congjun Zhu、Jie Dong、Xueting Liu、Liuzhou Gao、Yue Zhao、Jin Xie、Shuhua Li、Chengjian ZhuDOI:10.1002/anie.202005749日期:2020.7.27In this Communication, we report an unprecedented β‐regioselective radical inverse hydroboration (compared with ionic hydroboration) of α,β‐unsaturated amides with NHC‐BH3 enabled by photoredox catalysis. Density functional theory (DFT) calculations show that the unique photoredox cycle is a key factor to control the β‐regioselective radical hydroboration, by lowering the energy barrier in comparison
-
SYNTHESIS OF MONOFUNCTIONAL THIURAM ACCELERATOR申请人:The Goodyear Tire & Rubber Company公开号:US20210198194A1公开(公告)日:2021-07-01The present invention provides a route for synthesizing monofunctional thiuram compounds that is safe, environmentally friendly, and cost effective. This method specifically involves synthesizing a monofunctional thiuram by (1) reacting a tetraorganylthiuram disulfide with an organyl mercaptan to produce the monofunctional thiuram and a dithiocarbamate metal salt or a dithiocarbamate metalloid salt under basic conditions, (2) separating the monofunctional thiuram in an organic phase from the dithiocarbamate metal salt or the dithiocarbamate metalloid salt in an aqueous phase, and (3) recovering the monofunctional thiuram from the aqueous phase. The monofunctional thiuram compounds made in accordance with this invention are of particular value as accelerators for use in the vulcanization of rubber. The use of these monofunctional thiuram compounds as accelerators provides good cure rates and as well as good scorch safety.
-
Reactions of Substituted Indoles with 2,3-Dichloro-1,4-naphthoquinone and Electrochemical Properties of Some 2,3-Substituted 1,4-Naphthoquinones作者:C. Ibis、S. Sahinler Ayla、D. Tulegenova、H. BaharDOI:10.1134/s1070428019040213日期:2019.4The reactions of 2,3-dichloro-1,4-naphthoquinone with some indoles and thiols were investigated. The resulting nucleophilic substitution products were characterized by spectroscopic methods (FT-IR, 1H and 13C NMR, MS) and microanalysis. The effects of polar and nonpolar solvents on the electronic absorption spectra and electrochemical properties of some newly synthesized compounds were also studied研究了2,3-二氯-1,4-萘醌与一些吲哚和硫醇的反应。所得亲核取代产物通过光谱方法(FT-IR,1 H和13 C NMR,MS)和微量分析进行表征。还研究了极性和非极性溶剂对一些新合成化合物的电子吸收光谱和电化学性质的影响。
-
A high yielding, one-pot synthesis of S,S-dialkyl dithiocarbonates through the corresponding thiols using Mitsunobu’s reagent作者:A. K. Chaturvedi、D. Chaturvedi、N. Mishra、V. MishraDOI:10.1007/bf03246060日期:2010.9A novel Mitsunobu-based technique has been developed for the synthesis of a variety of symmetrical and unsymmetrical S,S-dialkyl dithiocarbonates from various corresponding primary, secondary and tertiary thiols using gaseous carbon dioxide, in good to excellent yields.
表征谱图
-
氢谱1HNMR
-
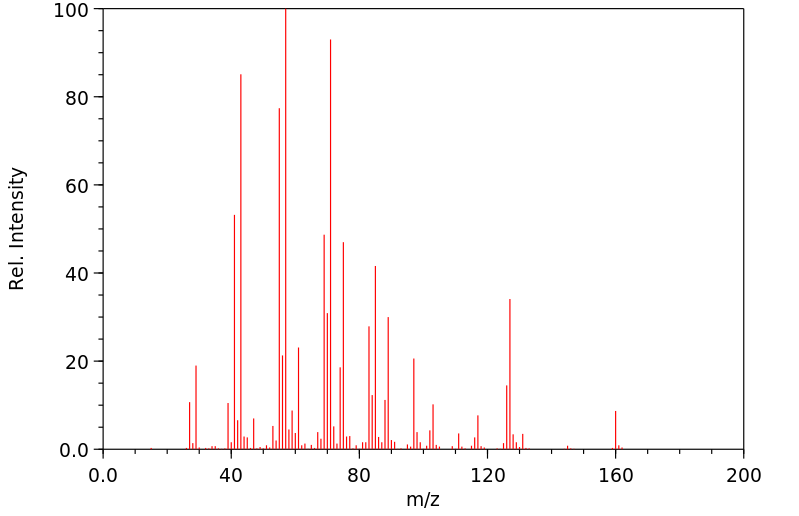
质谱MS
-
碳谱13CNMR
-
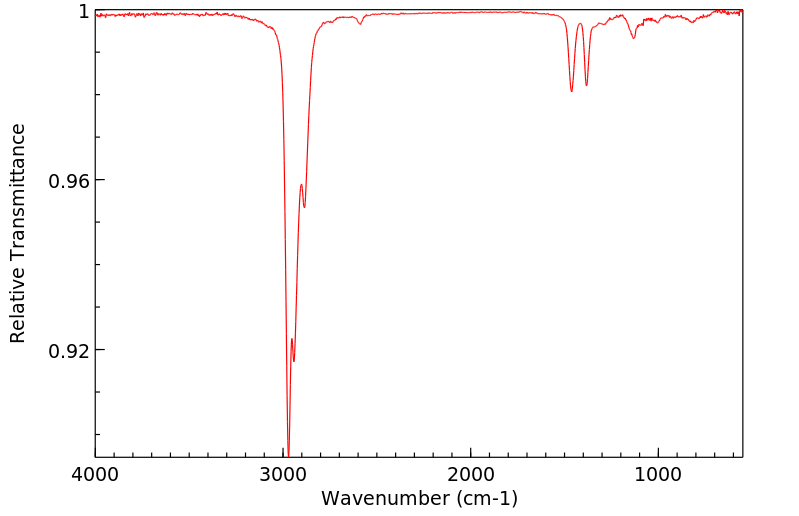
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
铜,丙烷-2-硫醇
铅,丙烷-1-硫醇
苏-(2R,4R)-戊二硫醇
羟基-乙醛
硫甘油
癸烷-2-硫醇
甲硫醇铅
甲硫醇钠
甲硫醇-d4
甲硫醇-S-d
甲硫醇
甲三硫醇
环辛硫醇
环戊硫醇
环戊基甲硫醇
环庚烷-1,1-二硫醇
环己硫醇
环己烷-1,1-二硫醇
环己基甲硫醇
环十二烷硫醇
环丙硫醇
环丙基甲硫醇
环丁硫醇
油烯基硫醇
氟甲硫醇
氘代甲硫醇-D3
正十四烷基硫醇
末端脱氧核苷酸转移酶
戊赤藓四硫醇
戊烷-3-硫醇
异戊硫醇
异戊烯基硫醇
异丙硫醇
异丁硫醇
庚-3-烯-4-硫醇
己-2-烯-1-硫醇
巯基甲烷-13C
巯基乙醛
巯基乙胺氢溴酸盐
巯基乙胺
巯基-十一胺盐酸盐
壬烷-2-硫醇
吡啶,2-(戊基硫代)-,1-氧化
叔壬基硫醇
叔十六硫醇
叔十二烷硫醇
叔丁基硫醇
反式-2-丁烯-1-硫醇
双(巯基环戊烷)四氯化钛
半胱胺盐酸盐