sodium 2-oxobutyrate | 2013-26-5
中文名称
——
中文别名
——
英文名称
sodium 2-oxobutyrate
英文别名
sodium 2-ketobutyrate;sodium 2-oxobutanoate;sodium;2-oxobutanoate
CAS
2013-26-5
化学式
C4H5O3*Na
mdl
——
分子量
124.072
InChiKey
SUAMAHKUSIHRMR-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,目前没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):-4.28
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:57.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2918300090
-
RTECS号:ET5955900
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:密封,在2°C至-8°C下保存
SDS
2-氧代丁酸钠 修改号码:5
模块 1. 化学品
产品名称: Sodium 2-Oxobutyrate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-氧代丁酸钠
百分比: >98.0%(T)
CAS编码: 2013-26-5
俗名: 2-Oxobutyric Acid Sodium Salt , Sodium 2-Ketobutyrate , 2-Ketobutyric Acid
Sodium Salt
分子式: C4H5NaO3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
2-氧代丁酸钠 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
2-氧代丁酸钠 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: scu-mus LD50:2960 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: ET5955900
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2-氧代丁酸钠 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Sodium 2-Oxobutyrate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2-氧代丁酸钠
百分比: >98.0%(T)
CAS编码: 2013-26-5
俗名: 2-Oxobutyric Acid Sodium Salt , Sodium 2-Ketobutyrate , 2-Ketobutyric Acid
Sodium Salt
分子式: C4H5NaO3
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
2-氧代丁酸钠 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
2-氧代丁酸钠 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: scu-mus LD50:2960 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: ET5955900
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
2-氧代丁酸钠 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
用途: 用于生化研究,是乳酸脱氢酶的底物,也常用于制备诊断试剂。
反应信息
-
作为反应物:参考文献:名称:A New Family of
D -2-Hydroxyacid Dehydrogenases That ComprisesD -Mandelate Dehydrogenases and 2-Ketopantoate Reductases摘要:通过活性染色程序和PCR分离粪肠球菌IAM10071的d-扁桃酸脱氢酶(d-ManDH)基因,并在大肠杆菌细胞中表达。该重组酶对各种具有大量疏水侧链的2-酮酸底物,特别是C3支链底物,如苯甲酰甲酸和2-酮异戊酸,表现出高催化活性,并对NADH和NAD+表现出严格的辅酶特异性。它与已知的 NADP 依赖性 2-酮泛解酸还原酶 (KPR) 显示出显着的序列相似性。这些结果表明,d-ManDH 与 KPR 一起构成了一个新的 d-2-羟基酸脱氢酶家族,其作用于 C3 支链 2-酮酸底物,对辅酶和底物具有各种特异性。DOI:10.1271/bbb.70827 -
作为产物:参考文献:名称:一种制备2-丁酮酸钠盐的工艺方法摘要:本发明公开了一种制备2‑丁酮酸钠盐的工艺方法,包括以下步骤:S1:以甲基丙二酸二乙酯或甲基丙二酸二甲酯为原料,与草酸二乙酯或草酸二甲酯在适量碱性化合物催化下反应得到1‑羰基丙烷‑1,2,2‑三羧酸酯中间体;S2:将所述S1中得到的1‑羰基丙烷‑1,2,2‑三羧酸酯中间体与水在适量酸性化合物催化下反应得到2‑丁酮酸;S3:将步骤S2中得到的2‑丁酮酸与碱性化合物反应得到2‑丁酮酸钠盐。其路线设计合理,安全可靠,成本较低,能够量化生产。公开号:CN111138269B
文献信息
-
Biocatalytic Construction of Quaternary Centers by Aldol Addition of 3,3-Disubstituted 2-Oxoacid Derivatives to Aldehydes作者:Roser Marín-Valls、Karel Hernández、Michael Bolte、Teodor Parella、Jesús Joglar、Jordi Bujons、Pere ClapésDOI:10.1021/jacs.0c09994日期:2020.11.183-methyl-2-oxobutanoate hydroxymethyltransferase from E. coli (KPHMT) and variants thereof. The 3,3,3-trisubstituted 2-oxoacids thus produced were converted into 2-oxolactones and 3-hydroxy acids and directly to ulosonic acid derivatives, all bearing gem-dialkyl, gem-cycloalkyl, and spirocyclic quaternary centers. In addition, some of these reactions use a single enantiomer from racemic nucleophiles to afford
-
Exploration of Aldol Reactions Catalyzed by Stereoselective Pyruvate Aldolases with 2‐Oxobutyric Acid as Nucleophile作者:V. Laurent、A. Uzel、V. Hélaine、L. Nauton、M. Traïkia、T. Gefflaut、M. Salanoubat、V. de Berardinis、M. Lemaire、C. Guérard‐HélaineDOI:10.1002/adsc.201900128日期:——family PF03328, recently described to be able to use hydroxypyruvic acid as nucleophile substrate, were shown to also catalyse aldol adducts formation from 2‐oxobutyric acid. A 1H NMR‐based assay was used to screen 21 aldolases for their activity towards 2‐oxobutyric acid and to predict their stereoselectivity at position 3 of the aldol adducts. The best biocatalysts were then proved to be strictly (3S)‐selective
-
Metallkomplexe von Farbstoffen, XII [1], Halbsandwich-Komplexe von Ruthenium, Cobalt, Rhodium, Iridium mit Iminocarboxylaten aus 4-(4′-Nitrophenylazo)anilin (Disperse Orange 3) oder 4,4′-Diaminoazobenzol und 2-Oxocarboxylaten. Metal Complexes of Dyes, XII作者:Frank Kühlwein、Wolfgang BeckDOI:10.1002/zaac.201000162日期:2010.11: Beck, Wolfgang, wbe@cup.uni-muenchen.de merged with this user on 29-May-2008 by Knapp, Jeanette (Beck, Wolfgang): Beck, Wolfgang, wbe@cup.uni-muenchen.de 于 29-May-2008 由 Knapp, Jeanette (Beck, Wolfgang) 与该用户合并
-
Quinoline tricyclic derivatives. Design, synthesis and evaluation of the antiviral activity of three new classes of RNA-dependent RNA polymerase inhibitors作者:Antonio Carta、Irene Briguglio、Sandra Piras、Paola Corona、Giampiero Boatto、Maria Nieddu、Paolo Giunchedi、Maria Elena Marongiu、Gabriele Giliberti、Filippo Iuliano、Sylvain Blois、Cristina Ibba、Bernardetta Busonera、Paolo La CollaDOI:10.1016/j.bmc.2011.10.009日期:2011.12CVB-5, Reo-1 and RSV. However, derivatives belonging to all classes showed activity against BVDV. Among the most potent were the bis-triazoloquinoline 1m, the imidazoquinolines 2e and 2h, and the pyridoquinoxalines 4h, 4j and 5n (EC50 range 1–5 μM). When tested in a replicon assay, compound 2h was the sole derivative to also display anti-HCV activity (EC50 = 3.1 μM). In enzyme assays, 1m, 2h, 5m and 5n在这项研究中,合成了三类新的线性N-三环化合物,它们是由喹啉核与1,2,3-三唑,咪唑或吡嗪缩合而得到的,获得了三唑并[4,5- g ]喹啉,咪唑并[4]。分别是,5-5- g喹啉和吡啶并[2,3- g ]喹喔啉。标题化合物中的细胞毒性和抗病毒活性的基于细胞的测定中测试抗RNA病毒代表三个属的的黄病毒科家族,即BVDV(瘟病毒),YFV(黄病毒属)和HCV(丙型肝炎病毒属)。喹啉衍生物也针对含有单链,无论正义(单链RNA其他RNA病毒科的代表测试+)或负义(RNA - ,和双链基因组(双链RNA),以及针对两个代表) DNA病毒家族。尽管对CVB-5,Reo-1和RSV具有选择性活性,但一些喹啉类药物显示中等程度的活性。但是,属于所有类别的衍生物均显示出对BVDV的活性。最有效的是双三唑并喹啉1m,咪唑并喹啉2e和2h以及吡啶并喹喔啉4h,4j和5n(EC 50范围1-5μM)。当在复制子
-
Absolute configuration of the product of the acetolactate synthase reaction by a novel method of analysis using acetolactate decarboxylase作者:David H. G. Crout、Edward R. Lee、Daniel L. RathboneDOI:10.1039/p19900001367日期:——2-oxobutanoate were incubated with acetolactate synthase [acetolactate pyruvate lyase (carboxylating, EC 4.1.3.18)] in the presence of acetolactate decarboxylase [(S)-2-hydroxy-2-methyl-3-oxobutanoate carboxy-lyase, EC 4.1.1.5)]. The exclusive production of 3-hydroxypentan-2-one showed that the α-acetohydroxybutyrate (2-ethyl-2-hydroxy-3-oxobutanoate) produced by acetolactate synthase had the (S)-configuration
表征谱图
-
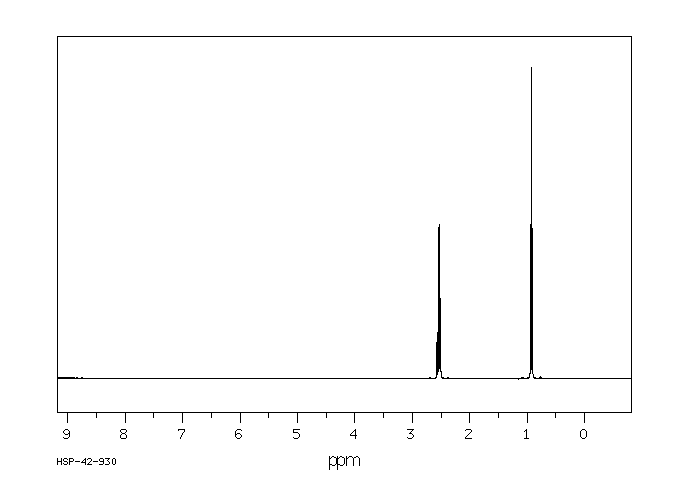
氢谱1HNMR
-
质谱MS
-
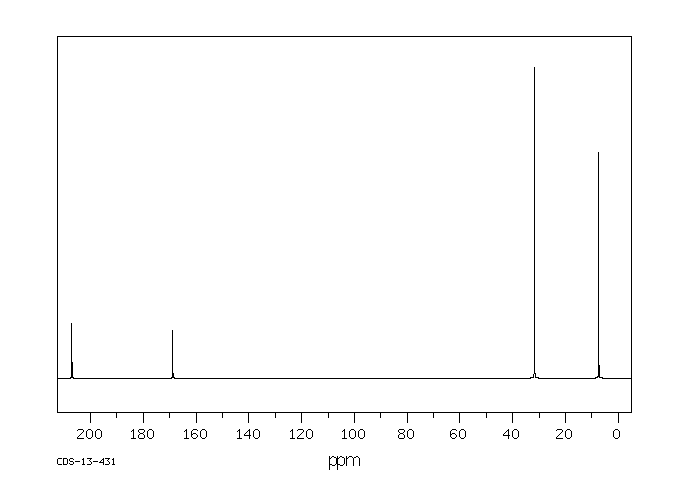
碳谱13CNMR
-
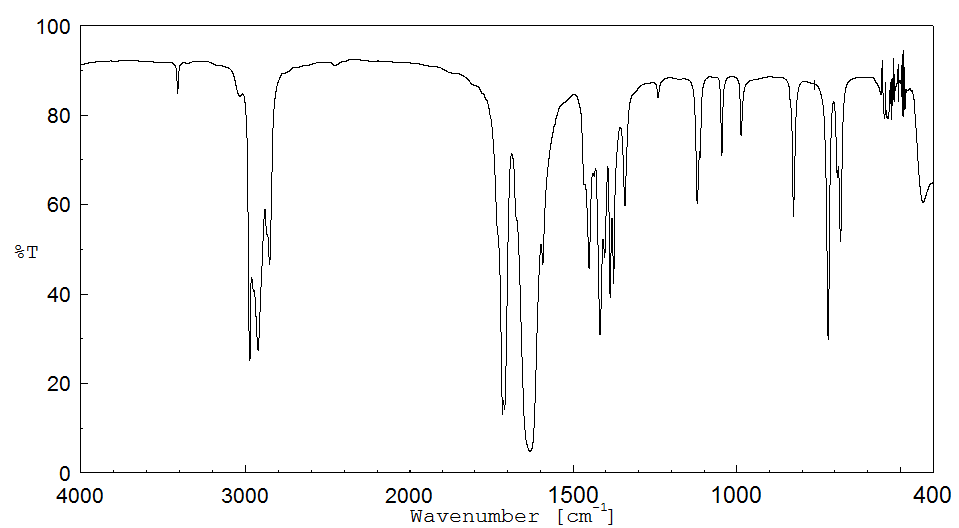
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
马来酰基乙酸
顺-3-己烯-1-丙酮酸
青霉酸
钠氟草酰乙酸二乙酯
醚化物
酮霉素
辛酸,2,4-二羰基-,乙基酯
草酸乙酯钠盐
草酰乙酸二乙酯钠盐
草酰乙酸二乙酯
草酰乙酸
草酰丙酸二乙酯
苯乙酰丙二酸二乙酯
苯丁酸,b-羰基-,2-丙烯基酯
聚氧化乙烯
羟基-(3-羟基-2,3-二氧代丙基)-氧代鏻
磷酸二氢2-{(E)-2-[4-(二乙胺基)-2-甲基苯基]乙烯基}-1,3,3-三甲基-3H-吲哚正离子
碘化镝
硬脂酰乙酸乙酯
甲氧基乙酸乙酯
甲氧基乙酰乙酸酯
甲基氧代琥珀酸二甲盐
甲基4-环己基-3-氧代丁酸酯
甲基4-氯-3-氧代戊酸酯
甲基4-氧代癸酸酯
甲基4-氧代月桂酸酯
甲基4-(甲氧基-甲基磷酰)-2,2,4-三甲基-3-氧代戊酸酯
甲基3-羰基-2-丙酰戊酸酯
甲基3-氧代十五烷酸酯
甲基2-氟-3-氧戊酯
甲基2-氟-3-氧代己酸酯
甲基2-氟-3-氧代丁酸酯
甲基2-乙酰基环丙烷羧酸酯
甲基2-乙酰基-4-甲基-4-戊烯酸酯
甲基2-乙酰基-2-丙-2-烯基戊-4-烯酸酯
甲基2,5-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代戊酸酯
甲基2,4-二氟-3-氧代丁酸酯
甲基1-异丁酰基环戊烷羧酸酯
甲基1-乙酰基环戊烷羧酸酯
甲基1-乙酰基环丙烷羧酸酯
甲基1-乙酰基-2-乙基环丙烷羧酸酯
甲基(2Z,4E,6E)-2-乙酰基-7-(二甲基氨基)-2,4,6-庚三烯酸酯
甲基(2S)-2-甲基-4-氧代戊酸酯
甲基(1S,2R)-2-乙酰基环丙烷羧酸酯
甲基(1R,2R)-2-乙酰基环丙烷羧酸酯
瑞舒伐他汀杂质
瑞舒伐他汀杂质
环氧乙烷基甲基乙酰乙酸酯
环戊戊烯酸,Β-氧代,乙酯