2,3-二溴丙酸甲酯 | 1729-67-5
中文名称
2,3-二溴丙酸甲酯
中文别名
一缩二乙二醇一(2-乙基已基)醚;二甘醇(2-乙己基)醚;2-乙己基卡必醇;二甘醇一(2-乙己基)醚;二乙二醇单辛醚;一缩二乙二醇单(2-乙基已基)醚;D-哌可酸;2-{(2-[(2-乙基己基)氧基]乙氧基}乙醇;二甘醇单(2-乙己基)醚
英文名称
Methyl 2,3-dibromopropionate
英文别名
2,3-dibromo-propionic acid methyl ester;methyl 2,3-dibromopropanoate
CAS
1729-67-5
化学式
C4H6Br2O2
mdl
MFCD00017882
分子量
245.898
InChiKey
ROXQOUUAPQUMLN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:83-86 °C/10 mmHg (lit.)
-
密度:1.944 g/mL at 20 °C (lit.)
-
闪点:-33 °C
-
LogP:2
-
保留指数:1070
-
稳定性/保质期:
常温常压下稳定,避免与强酸接触以及氧化物的生成。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S16,S26,S36
-
危险类别码:R36/37
-
WGK Germany:3
-
危险品运输编号:UN 2398 3/PG 2
-
海关编码:2915900090
-
危险类别:6.1
-
包装等级:III
-
储存条件:请将容器密封保存,并储存在阴凉、干燥的地方。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Methyl 2,3-dibromopropionate
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 2,3-dibromopropionate
CAS number: 1729-67-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C4H6Br2O2
Molecular weight: 245.9
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Methyl 2,3-dibromopropionate
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 2,3-dibromopropionate
CAS number: 1729-67-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
This product should be handled only by, or under the close supervision of, those properly qualified
Handling:
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C4H6Br2O2
Molecular weight: 245.9
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3-二溴丙酸 2,3-dibromopropionic acid 600-05-5 C3H4Br2O2 231.872 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (S)-甲基-2-溴-3-羟基丙酸甲酯 methyl 2-bromo-3-hydroxypropanoate 7691-28-3 C4H7BrO3 183.002 —— methyl α-bromo-β-fluoropropionate 1537-52-6 C4H6BrFO2 184.993
反应信息
-
作为反应物:描述:2,3-二溴丙酸甲酯 在 sodium azide 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 以70%的产率得到methyl 2-azidoacrylate参考文献:名称:通过β,γ-C,S硫-迈克尔加成反应化学合成N-末端半胱氨酸硫酯摘要:脱氢丙氨酸(ΔAla)是高度亲电的残基,可与硫亲核试剂有效反应,提供半胱氨酰类似物。本文中,我们报告了基于β,γ-C,S硫醇-迈克尔加成法的N端半胱氨酸硫酯的有效合成方法,适用于S,N-酰基转移。发现基于离子方法和基于自由基的方法对于该过程都是有效的。DOI:10.1021/acs.orglett.9b01013
-
作为产物:描述:参考文献:名称:溴阳离子有机催化还原的光谱和计算研究:加速立体选择性二溴化方案**摘要:报道了一种新开发的使用胺有机催化剂从N-溴代琥珀酰亚胺 (NBS) 中原位还原阳离子溴的方法。中间体试剂及其反应性已通过光谱和计算方法得到了很好的表征。NBS 分子的战略结构变化可以引起足够的极性反转,以加速需要两个“Br”合成子的二溴化反应。这已进一步用于具有广泛底物范围的烯烃的加速二溴化反应。DOI:10.1002/chem.202300675
-
作为试剂:描述:2-amino-5-methyl-benzoic acid ethyl ester; thiocyanate 在 2,3-二溴丙酸甲酯 作用下, 反应 2.5h, 生成 ethyl 7-methyl-5-oxo-1,2-dihydro-[1,3]thiazolo[3,2-a]quinazoline-2-carboxylate;hydrobromide参考文献:名称:Gakhar, H. K.; Kiran, Sushma; Gupta, Shashi Bhushan, Journal of the Indian Chemical Society, 1982, vol. 59, # 5, p. 666 - 667摘要:DOI:
文献信息
-
Efficient Synthesis of Highly Functionalized Cyclic Aminimides作者:Bongjin Moon、Sangbae Han、Dohyung KimDOI:10.1021/ol051270v日期:2005.7.1[reaction: see text]. Simple condensation reactions of various alpha,beta-epoxy or alpha,beta-aziridinyl methyl esters with 1,1-dialkyl hydrazines provided cyclic aminimides (1,1-dialkyl-3-oxopyrazolidines) with a heteroatom substituent at the 4-position in good yields. The reaction proceeds smoothly, without any coreagent, providing the product as an easily isolable precipitate. The reaction is expected
-
[EN] ROR-GAMMA INHIBITORS<br/>[FR] INHIBITEURS DE ROR-GAMMA申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2019063748A1公开(公告)日:2019-04-04The present invention relates to compounds of formula I and pharmaceutical compositions comprising compounds of formula I. Compounds of Formula I are useful in treatment of inflammatory, metabolic or autoimmune diseases which are mediated by RORy.本发明涉及公式I的化合物和包含公式I化合物的药物组合物。公式I的化合物在治疗由RORγ介导的炎症性、代谢性或自身免疫性疾病方面是有用的。
-
Reaction of N-Haloamide. XX. Bromo-formyloxylation of α, β-Unsaturated Esters with N, N-Dibromobenzenesulfonamide and Formic Acid作者:YOSHIO UENO、AKEMI YAMASAKI、HIROMI TERAUCHI、SHOJI TAKEMURADOI:10.1248/cpb.22.1646日期:——α, β-Unsaturated esters were made to react with N, N-dibromobenzenesulfonamide and formic acid in chloroform to give bromo-formyloxyesters. Methyl acrylate (1a), ethyl crotonate (2a), ethyl trans-cinnamate (3a), methyl tiglate (4a), methyl methacrylate (5a), and methyl phenylacrylate (6a) gave methyl 2-bromo-3-formyloxypropionate (1b), ethyl erythro-2-bromo-3-formyloxybutyrate (2b), ethyl erythro-2-bromo-3-formyloxy-3-phenylpropionate (3b), methyl erythro-2-bromo-3-formyloxy-2-methylbutyrate (4b), a mixture of methyl 3-bromo-2-formyloxy-2-methylpropionate (5b) and methyl 2-bromo-3-formyloxy-2-methylpropionate (5c), and methyl 3-bromo-2-formyloxy-2-phenylpropionate (6b) respectively in good yields.α, β-不饱和酯与N, N-二溴苯磺酰胺和甲酸在氯仿中反应,生成溴代甲酰氧基酯。甲基丙烯酸酯(1a)、乙基巴豆酸酯(2a)、乙基反式肉桂酸酯(3a)、甲基惕格酸酯(4a)、甲基甲基丙烯酸酯(5a)和甲基苯基丙烯酸酯(6a)分别以良好的产率生成甲基2-溴-3-甲酰氧基丙酸酯(1b)、乙基erythro-2-溴-3-甲酰氧基丁酸酯(2b)、乙基erythro-2-溴-3-甲酰氧基-3-苯基丙酸酯(3b)、甲基erythro-2-溴-3-甲酰氧基-2-甲基丁酸酯(4b)、甲基3-溴-2-甲酰氧基-2-甲基丙酸酯(5b)和甲基2-溴-3-甲酰氧基-2-甲基丙酸酯(5c)的混合物以及甲基3-溴-2-甲酰氧基-2-苯基丙酸酯(6b)。
-
Hybridized and isosteric analogues of N 1 -acetyl- N 4 -dimethyl-piperazinium iodide (ADMP) and N 1 -phenyl- N 4 -dimethyl-piperazinium iodide (DMPP) with central nicotinic action作者:Dina Manetti、Alessandro Bartolini、Pier Andrea Borea、Cristina Bellucci、Silvia Dei、Carla Ghelardini、Fulvio Gualtieri、Maria Novella Romanelli、Serena Scapecchi、Elisabetta Teodori、Katia VaraniDOI:10.1016/s0968-0896(98)00259-4日期:1999.3tertiary bases (2, 4) with arecoline (5) and arecolone (6) or by isosteric substitution of the phenyl ring of DMPP, has been synthesized. Hybridization afforded compounds that, both as tertiary bases and as iodomethylates, have no affinity for the nicotinic receptor. On the contrary, isosteric substitution gave compounds that maintain affinity for the receptor; among them, two tertiary bases (37, 38)
-
[EN] QUINOLIN-, ISOQUINOLIN-, AND QUINAZOLIN-OXYALKYLAMIDES AND THEIR USE AS FUNGICIDES<br/>[FR] OXYALKYLAMIDES DE QUINOLINE, D'ISOQUINOLINE ET DE QUINAZOLINE ET LEUR UTILISATION COMME FONGICIDES申请人:SYNGENTA LTD公开号:WO2004047538A1公开(公告)日:2004-06-10Fungicidal compounds of the general formula (1) wherein one of X and Y is N or N-oxide and the other is CR or both of X and Y are N.通用公式(1)中的杀真菌化合物,其中X和Y中的一个是N或N-氧化物,另一个是CR,或者X和Y都是N。
表征谱图
-
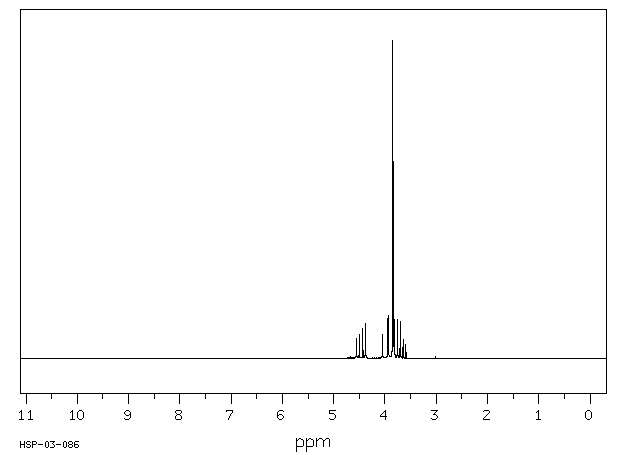
氢谱1HNMR
-
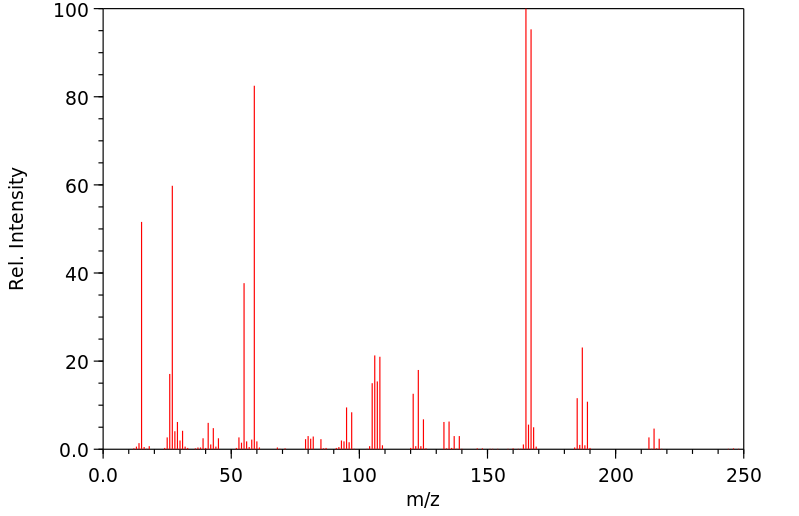
质谱MS
-
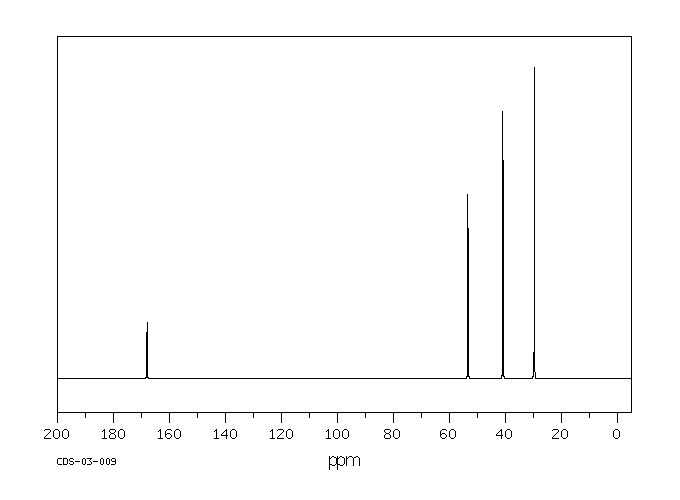
碳谱13CNMR
-
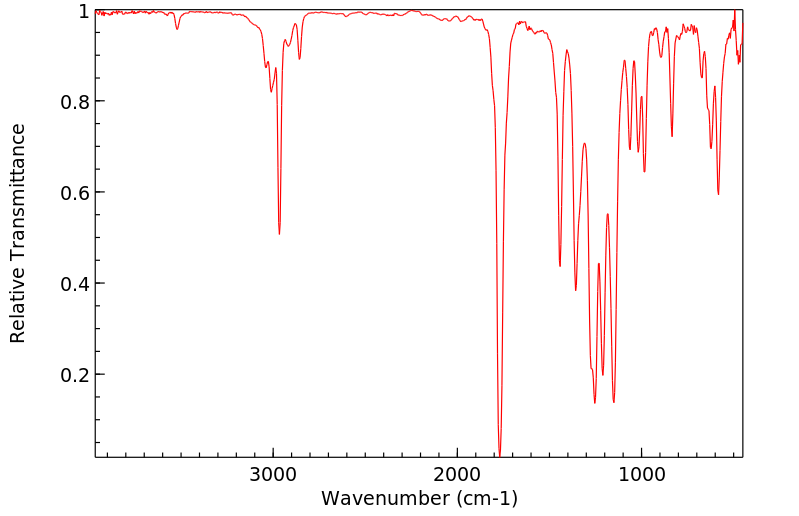
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸