N-(4-乙氧基苯基)苯甲酰胺 | 15437-14-6
中文名称
N-(4-乙氧基苯基)苯甲酰胺
中文别名
——
英文名称
N-(4-ethoxyphenyl)benzamide
英文别名
——
CAS
15437-14-6
化学式
C15H15NO2
mdl
MFCD00087847
分子量
241.29
InChiKey
LOKFUBTWUGZRLA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:18
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:38.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2924299090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-(2-氨基苯基)苯甲酰胺 2'-Aminobenzanilid 721-47-1 C13H12N2O 212.251 非那西丁 4-ethoxyacetanilide 62-44-2 C10H13NO2 179.219
反应信息
-
作为反应物:描述:参考文献:名称:苯碘双(三氟乙酸盐)介导的富电子 N-苯基苯甲酰胺的分子内氧化偶联摘要:N-(4-烷氧基-苯基)和N-(4-乙酰氨基-苯基)苯甲酰胺的分子内氧化C-O偶联是在无金属条件下通过使用苯基碘双(三氟乙酸盐)作为氧化剂和TMSOTf作为催化剂实现的。该反应以高产率提供苯并恶唑产物。DOI:10.1055/s-0031-1290680
-
作为产物:描述:参考文献:名称:利用 PhNCO 从 N-(2-氨基苯基)苯甲酰胺合成仲酰胺摘要:我们成功开发了一种利用容易获得的N- (2-氨基苯基)苯甲酰胺和异氰酸苯酯合成仲酰胺的有效方法。值得注意的是,离去基团可以很容易地恢复为羰基化的N-杂环,这在制药应用中具有显着的相关性。该策略的关键涉及异氰酸苯酯的连续亲核/分子内加成过程,然后进行转酰胺基化。此外,该方案还具有原子经济性、实用性、一锅两步反应以及底物制备简便等特点。DOI:10.1039/d3nj04995g
文献信息
-
Direct Amidation of Esters by Ball Milling**作者:William I. Nicholson、Fabien Barreteau、Jamie A. Leitch、Riley Payne、Ian Priestley、Edouard Godineau、Claudio Battilocchio、Duncan L. BrowneDOI:10.1002/anie.202106412日期:2021.9.27The direct mechanochemical amidation of esters by ball milling is described. The operationally simple procedure requires an ester, an amine, and substoichiometric KOtBu and was used to prepare a large and diverse library of 78 amide structures with modest to excellent efficiency. Heteroaromatic and heterocyclic components are specifically shown to be amenable to this mechanochemical protocol. This
-
Direct Hydrogenation of a Broad Range of Amides under Base-free Conditions using an Efficient and Selective Ruthenium(II) Pincer Catalyst作者:Zheng Wang、Yong Li、Qing-bin Liu、Gregory A. Solan、Yanping Ma、Wen-Hua SunDOI:10.1002/cctc.201700952日期:2017.11.23Amide hydrogenation: A catalyst loading of between 0.1–1 mol % of the RuII complex B catalyzes the hydrogenation of a broad range of amides under base-free conditions efficiently and selectively to afford the corresponding alcohols and amines in high yields.酰胺加氢:Ru II络合物 B的负载量为0.1–1 mol%,可在无碱条件下高效,选择性地催化各种酰胺的加氢反应,从而以高收率提供相应的醇和胺。
-
Direct Synthesis of Amides from Coupling of Alcohols and Amines Catalyzed by Ruthenium(II) Thiocarboxamide Complexes under Aerobic Conditions作者:Elangovan Sindhuja、Rengan Ramesh、Sundarraman Balaji、Yu LiuDOI:10.1021/om500556b日期:2014.8.25the ruthenium(II) thiocarboxamide complexes were generated as highly efficient catalysts for synthesis of secondary or tertiary amides by coupling of amines and alcohols with low catalyst loading, and the maximum yield was obtained up to 97%. The coupling reaction can be readily carried out under mild aerobic conditions, and release of water is the only byproduct. Further, the effect of substituents从1当量的钌的反应合成了四种通式[RuClCO(AsPh 3)2(L)]的八面体钌(II)硫代羧酰胺配合物(L = N-取代吡啶-2-硫代羧酰胺)前体[RuHClCO(AsPh 3)3在碱存在下于回流的乙醇中用1当量的硫代羧酰胺配体制备]。所有新的络合物均已通过元素分析,IR,UV-vis和NMR光谱法得到了充分表征。通过X射线晶体学测定所有配合物的分子结构,这证实了硫代羧酰胺的配位模式并揭示了Ru离子周围存在扭曲的八面体几何形状。所有的钌(II)硫代羧酰胺配合物都是通过胺和醇的偶联而以低催化剂负载量合成的,作为合成仲或叔酰胺的高效催化剂,其最高收率高达97%。偶合反应可以在温和的有氧条件下容易地进行,并且水的释放是唯一的副产物。此外,配体的取代基,溶剂,研究了反应温度,时间和催化剂负载量对配合物催化活性的影响。提出了一种可能的机理,用于通过半胱氨酸作为中间体通过醇氧化为醛来合成酰胺。
-
Switching from Biaryl Formation to Amidation with Convoluted Polymeric Nickel Catalysis作者:Abhijit Sen、Raghu N. Dhital、Takuma Sato、Aya Ohno、Yoichi M. A. YamadaDOI:10.1021/acscatal.0c03888日期:2020.12and we confirmed that it is consistent with Ni K-edge EXAFS. The Suzuki–Miyaura-type coupling of aryl halides and arylboronic esters proceeded using P4VP-NiCl2 (0.1 mol % Ni) to give the corresponding biaryl compounds in up to 94% yield. Surprisingly, when the same reaction of aryl halides and arylboronic acid/ester was carried out in the presence of amides, the amidation proceeded predominantly to通过聚(4-乙烯基吡啶)(P4VP)和氯化镍的分子卷积制备了稳定,可重复使用且不溶的聚(4-乙烯基吡啶)镍催化剂(P4VP-NiCl 2)。我们基于元素分析和Ni K-edge XANES提出了前催化剂中Ni中心的配位结构,并证实它与Ni K-edge EXAFS一致。使用P4VP-NiCl 2进行芳基卤化物和芳基硼酸酯的Suzuki-Miyaura型偶联(0.1mol%Ni)得到相应的联芳基化合物,产率高达94%。出人意料的是,当在酰胺存在下进行芳基卤化物和芳基硼酸/酯的相同反应时,酰胺化主要进行,从而以高达99%的产率得到相应的芳基酰胺。相反,在不存在芳基硼酸/酯的情况下,芳基卤化物和酰胺的反应没有进行。P4VP-NiCl 2成功地催化了内酰胺化反应,制备了菲啶酮。P4VP-NiCl 2被重复使用了五次,而催化活性没有明显损失。使用P4VP-NiCl 2有效合成药物,天然产物和
-
Effective Nitration of Anilides and Acrylamides by<i>tert</i>-Butyl Nitrite作者:Yi-fei Ji、Hong Yan、Qi-bai JiangDOI:10.1002/ejoc.201403510日期:2015.3[10% Cu(NO3)(2)3H(2)O] nitration of anilides was developed by using TBN (tert-butyl nitrite) as a nitrating reagent to give the corresponding nitro-substituted aromatic products in good to excellent yields. The use of TBN also led to the selective nitration of acrylamides at room temperature to afford only the (E) isomer of the nitration product. A series of anilides and acrylamides with a broad array
表征谱图
-
氢谱1HNMR
-
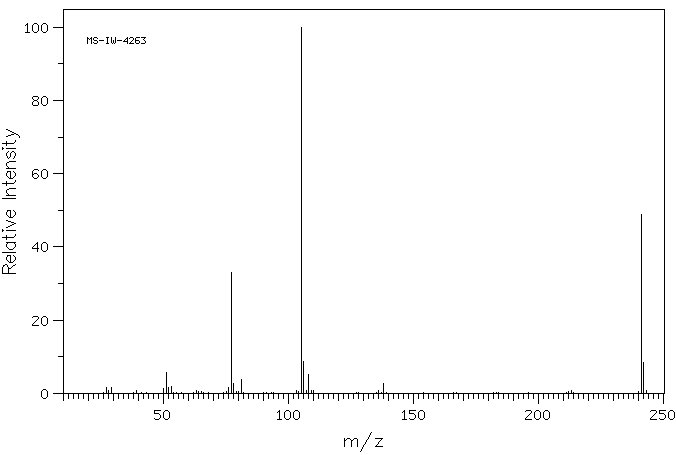
质谱MS
-
碳谱13CNMR
-
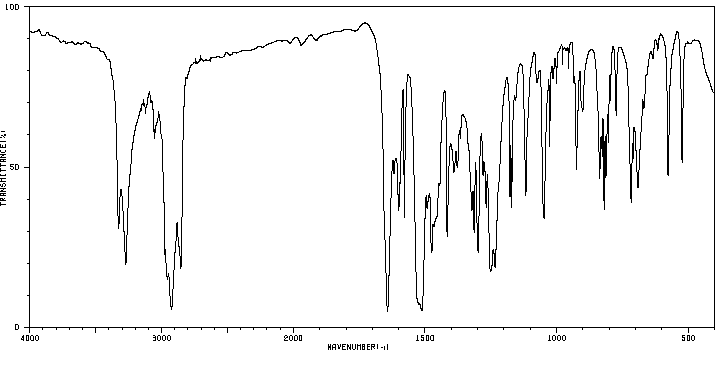
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫