N-(4-乙酰苯)-2,5-二甲基吡咯 | 83935-45-9
中文名称
N-(4-乙酰苯)-2,5-二甲基吡咯
中文别名
1-(4-乙酰基苯基)-2,5-二甲基吡咯;N-(4-乙酰苯基)-2,5-二甲基吡咯;1-(4-乙酰苯基)-2,5-二甲基吡咯
英文名称
1-(4-acetylphenyl)-2,5-dimethylpyrrole
英文别名
1-(4-(2,5-dimethyl-1H-pyrrol-1-yl)phenyl)ethan-1-one;4-(2,5-dimethyl-1H-pyrrol-1-yl)-acetophenone;1-(4-(2,5-Dimethyl-1H-pyrrol-1-yl)phenyl)ethanone;1-[4-(2,5-dimethylpyrrol-1-yl)phenyl]ethanone
CAS
83935-45-9
化学式
C14H15NO
mdl
MFCD00051816
分子量
213.279
InChiKey
QYTWQHUEXYLNLA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:109-113 °C
-
稳定性/保质期:
按规定使用和贮存的物质不会分解,也不会与氧化物反应。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.214
-
拓扑面积:22
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R20/21/22
-
海关编码:2933990090
-
储存条件:请将药品存放在密闭、阴凉、干燥的地方。
SDS
| Name: | N-(4-Acetylphenyl)-2 5-dimethylpyrrole 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 83935-45-9 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 83935-45-9 | N-(4-Acetylphenyl)-2,5-dimethylpyrrole | 98% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Light sensitive.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 83935-45-9: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 109 - 113 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Insoluble.
Specific Gravity/Density:
Molecular Formula: C14H15NO
Molecular Weight: 213.28
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 83935-45-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
N-(4-Acetylphenyl)-2,5-dimethylpyrrole - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 83935-45-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 83935-45-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 83935-45-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,5-二甲基-1-苯基吡咯 2,5-dimethyl-1-phenyl-1H-pyrrole 83-24-9 C12H13N 171.242 1-(4-溴苯基)-2,5-二甲基-1H-吡咯 1-(4-Bromo-phenyl)-2,5-dimethyl-1H-pyrrole 5044-24-6 C12H12BrN 250.138
反应信息
-
作为反应物:描述:N-(4-乙酰苯)-2,5-二甲基吡咯 在 吡啶 、 盐酸羟胺 作用下, 以 甲醇 为溶剂, 生成 4-(2,5-dimethyl-1H-pyrrol-1-yl)-acetophenone oxime参考文献:名称:Pyrrolylphenyl-substituted hydroxamic acid derivatives摘要:本文披露了以下化学方程式的吡咯基苯基取代羟肟酸衍生物:其中R代表氢、低碳烷基、卤素或低碳氧基;R1和R2独立地代表氢、低碳烷基或芳基;Y代表直链键、低碳烷基、低碳烯基、低碳二烯基、(硫代、亚砜基或磺代)-低碳烷基或氧-低碳烷基;Z代表其中R3代表氢或酰基;R4代表低碳烷基、C3-C7-环烷基、芳基或芳基-低碳烷基;或Z代表其中R3代表氢或酰基;R5代表低碳烷基、C3-C7-环烷基、芳基、芳基-低碳烷基、氨基或N-(单或双低碳烷基)-氨基;R6和R7代表氢或低碳烷基;以及其药用盐,前提是R3代表氢;这些化合物可用作选择性脂氧合酶抑制剂,其制备方法,包括所述化合物的制药组合物,以及使用所述化合物和包含所述化合物的制药组合物抑制脂氧合酶和治疗对脂氧合酶抑制有响应的哺乳动物疾病的方法。公开号:US05096919A1
-
作为产物:参考文献:名称:一种制备N-芳基吡咯化合物的方法摘要:本发明涉及一种制备N‑芳基吡咯化合物的方法。将含有不同取代基的呋喃,不同取代基的芳香胺和固体路易斯酸催化剂混合置于密闭反应器中,在一定催化条件下,制得具有不同取代基的N‑芳基吡咯化合物。催化反应条件的反应温度140‑210 oC。所述固体路易斯酸催化剂采用溶胶‑凝胶法制备获得,其中以Hf为核心金属元素,介孔分子筛SBA‑15为载体。该方法中催化剂制备简单、成本低,反应活性高,耐水性和结构稳定性好,催化反应产率高;同时,该类路易斯酸型催化剂不产生酸质子,避免高温下催化剂对设备腐蚀,反应后处理方便,催化剂可再生,对环境友好。公开号:CN111574424B
文献信息
-
Pyridine‐Stabilized Rhodium Nanoparticles in Ionic Liquids as Selective Hydrogenation and Transfer Hydrogenation Catalysts作者:Alejandro Serrano‐Maldonado、Erika Martin、Itzel Guerrero‐RíosDOI:10.1002/ejic.201900223日期:2019.6.30selectivity. Nitrobenzene was reduced to aniline with dihydrogen in the presence of RhNPs‐I with moderate activity. When the hydrogen source was formic acid‐Et3N azeotrope (transfer hydrogenation) the reaction was completed within minutes with high selectivity. Under transfer hydrogenation conditions, it was possible to apply the catalytic system RhNPs‐I in multistep processes for the generation of substituted在有机氢前体[Rh(µOMe)COD] 2下,在离子液体[BMIM] [BF 4 ](RhNPs-I至III)中用吡啶基配体稳定的铑纳米颗粒(RhNPs)是由二氢压力合成的。与不含配体的RhNPs-V相比,吡啶稳定的RhNPs尺寸更小,并且在苯乙酮加氢成1-苯基乙醇中表现出更高的活性和选择性。如果使用吡啶封端的RhNPs-I,则该系统可重复使用几次,而不会损失活性和选择性。在RhNPs-I存在下,硝基苯将硝基苯与二氢还原为苯胺适度活动。当氢源为甲酸-Et 3 N共沸物(转移氢化)时,反应可在数分钟内以高选择性完成。在转移加氢条件下,可以将催化体系RhNPs-I应用于多步过程中,通过硝基苯和苯甲醛的还原性N-烷基化反应生成取代的芳基胺。并通过硝基芳烃还原/ Paal-Knorr缩合合成取代的吡咯。
-
One-pot synthesis of N-substituted pyrroles from nitro compounds and 2,5-hexadione over a heterogeneous cobalt catalyst作者:Zheng Gong、Yu Lei、Peng Zhou、Zehui ZhangDOI:10.1039/c7nj01898c日期:——study, the one-pot heterocyclization of nitro compounds with 2,5-hexadione was studied for the synthesis of N-substituted pyrroles via a Paal–Knorr condensation process. The heterogeneous cobalt–nitrogen catalyst (Co–Nx/C-800-AT) was found to be active for this reaction with formic acid. Formic acid served as a hydrogen donor for the transfer hydrogenation, and also acted as an acid catalyst. More importantly
-
One-pot synthesis of cyclohexylamine and <i>N</i>-aryl pyrroles <i>via</i> hydrogenation of nitroarenes over the Pd<sub>0.5</sub>Ru<sub>0.5</sub>-PVP catalyst作者:Chandan Chaudhari、Katsutoshi Sato、Yasuyuki Ikeda、Kenji Terada、Naoya Abe、Katsutoshi NagaokaDOI:10.1039/d1nj00922b日期:——The direct synthesis of cyclohexylamine via the hydrogenation of nitrobenzene over monometallic (Pd, Ru or Rh) and bimetallic (PdxRu1−x) catalysts was studied. The Pd0.5Ru0.5-PVP catalyst was the most effective catalyst for this reaction. The catalyst can be reused and applied for the synthesis of N-aryl pyrroles and quinoxalines from nitrobenzenes.
-
Crystalline salicylic acid as an efficient catalyst for ultrafast Paal–Knorr pyrrole synthesis under microwave induction作者:Kioumars Aghapoor、Farshid Mohsenzadeh、Hossein Reza Darabi、Hani SayahiDOI:10.1007/s12039-021-01891-9日期:2021.6the viability of a wide range of crystalline aromatic and aliphatic carboxylic acids as organocatalysts has been investigated for solvent-free Paal–Knorr pyrrole synthesis under microwave activation. Among these potential catalysts, crystalline salicylic acid proved to be a remarkable catalyst because its efficiency remained high even under low microwave power irradiation or a shorter reaction time
-
Naturally occurring organic acids for organocatalytic synthesis of pyrroles via Paal–Knorr reaction作者:Farshid Mohsenzadeh、Hossein Reza Darabi、Mahsa Alivand、Kioumars Aghapoor、Yadollah BalavarDOI:10.1007/s11164-020-04260-2日期:2020.12In this study, common naturally occurring organic acids, namely oxalic, malonic, succinic, tartaric and citric acid (as safe, inexpensive, and biodegradable organocatalysts), have been employed for Paal–Knorr pyrrole synthesis. The organocatalyzed reaction proved to be effective in ethanol at 60 °C. However, the reaction rate is mainly dominated by the nature and position of functional groups on the
表征谱图
-
氢谱1HNMR
-
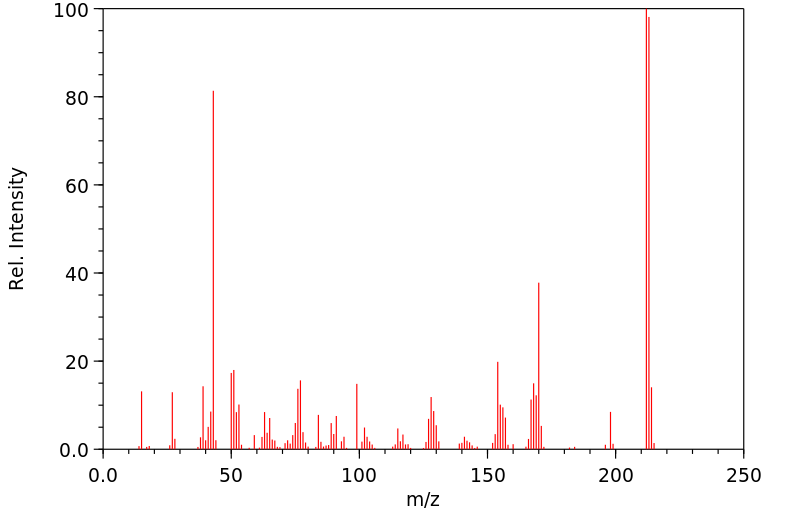
质谱MS
-
碳谱13CNMR
-
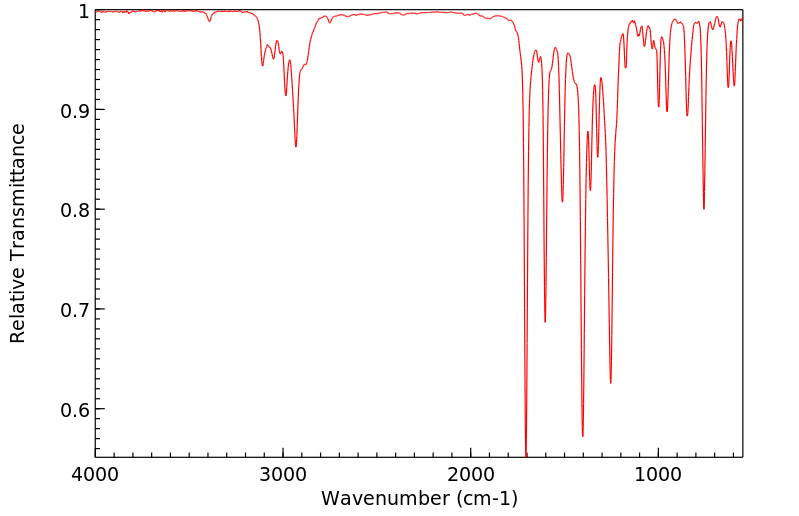
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷