棕榈酸异丁酯 | 110-34-9
中文名称
棕榈酸异丁酯
中文别名
十六酸2-甲基丙酯;十六酸-2-甲基丙酯
英文名称
hexadecanoic acid 2-methylpropyl ester
英文别名
isobutyl hexadecanoate;iso-butyl-palmitate;palmitic acid isobutyl ester;Palmitinsaeure-isobutylester;isobutyl palmitate;2-methylpropyl hexadecanoate
CAS
110-34-9
化学式
C20H40O2
mdl
MFCD00059289
分子量
312.536
InChiKey
OJIBJRXMHVZPLV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:19 °C
-
沸点:207 °C / 15mmHg
-
密度:0.86
-
闪点:196 °C
-
LogP:8.781 (est)
-
保留指数:2129
计算性质
-
辛醇/水分配系数(LogP):8.8
-
重原子数:22
-
可旋转键数:17
-
环数:0.0
-
sp3杂化的碳原子比例:0.95
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915709000
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:室温
SDS
Isobutyl Palmitate
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Isobutyl Palmitate
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Isobutyl Palmitate
Percent: >97.0%(GC)
CAS Number: 110-34-9
Synonyms: Palmitic Acid Isobutyl Ester
Chemical Formula: C20H40O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Isobutyl Palmitate
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:19°C (Freezing point)
207°C/2kPa
Boiling point/range:
Flash point: 196°C
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
0.86
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Isobutyl Palmitate
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Isobutyl Palmitate
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Isobutyl Palmitate
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Isobutyl Palmitate
Percent: >97.0%(GC)
CAS Number: 110-34-9
Synonyms: Palmitic Acid Isobutyl Ester
Chemical Formula: C20H40O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Isobutyl Palmitate
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colorless - Almost colorless
Colour:
Odour: No data available
pH: No data available
Melting point/freezing point:19°C (Freezing point)
207°C/2kPa
Boiling point/range:
Flash point: 196°C
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
0.86
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
Isobutyl Palmitate
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Isobutyl Palmitate
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
自组装材料和接触印刷材料
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 棕榈酸甲酯 hexadecanoic acid methyl ester 112-39-0 C17H34O2 270.456
反应信息
-
作为反应物:描述:参考文献:名称:The invention of radical reactions part XVIII. A convenient solution to the 1-carbon problem (R-CO2H → R-13CO2H)摘要:DOI:10.1016/s0040-4020(01)85980-9
-
作为产物:描述:参考文献:名称:The invention of radical reactions part XVIII. A convenient solution to the 1-carbon problem (R-CO2H → R-13CO2H)摘要:DOI:10.1016/s0040-4020(01)85980-9
文献信息
-
Certain substituted ureas, as modulators of kinase activity申请人:Mitchell A. Scott公开号:US20060270702A1公开(公告)日:2006-11-30Certain chemical entities chosen from compounds of Formula 1 and pharmaceutically acceptable salts, solvates, chelates, non-covalent complexes, and prodrugs thereof, are provided herein. Pharmaceutical compositions comprising at least one chemical entity and one or more pharmaceutically acceptable vehicles chosen from carriers, adjuvants, and excipients, are also provided herein. Methods of treating patients suffering from certain diseases and disorders responsive to angiogenic kinase modulation, which comprise administering to such patients an amount of at least one chemical entity effective to reduce signs or symptoms of the disease or disorder are disclosed. These diseases include cancer, including breast neoplasia, endometrial cancer, colon cancer, and neck squamous cell carcinoma. Methods of treatment include administering at least one chemical entity as a single active agent or administering such at least one chemical entity in combination with one or more other therapeutic agents. A method for determining the presence or absence of an angiogenic kinase in a sample comprising contacting the sample with at least one chemical entity under conditions that permit detection of activity of the angiogenic kinase, detecting a level of the activity of the angiogenic kinase, and therefrom determining the presence or absence of the angiogenic kinase in the sample.本文提供了从化合物1的化学实体和药用可接受的盐、溶剂化合物、螯合物、非共价复合物和前药中选择的某些化学实体。本文还提供了包括至少一种化学实体和一种或多种药用可接受载体(如载体、辅料和赋形剂)的药物组合物。公开了治疗对血管生成激酶调节敏感的某些疾病和疾病的方法,包括向这些患者施用至少一种化学实体的有效量以减少疾病或疾病症状的方法。这些疾病包括癌症,包括乳腺肿瘤、子宫内膜癌、结肠癌和颈部鳞状细胞癌。治疗方法包括将至少一种化学实体作为单一活性剂或将至少一种化学实体与一种或多种其他治疗剂结合使用的方法。一种用于确定样品中是否存在血管生成激酶的方法,包括将样品与至少一种化学实体接触在允许检测血管生成激酶活性的条件下,检测血管生成激酶活性水平,并从中确定样品中是否存在血管生成激酶。
-
[EN] TRPV4 RECEPTOR LIGANDS<br/>[FR] LIGANDS DU RÉCEPTEUR DU TRPV4申请人:UNIV UTAH RES FOUND公开号:WO2021102314A1公开(公告)日:2021-05-27Described are receptor ligands of transient receptor potential cation channel subfamily V member 4 (TRPV4), pharmaceutical compositions including the compounds, and methods of using the compounds and compositions for treating ocular disorders.描述了转瞬性受体电位阳离子通道亚家族V成员4(TRPV4)的受体配体,包括这些化合物的药物组合物,以及使用这些化合物和组合物治疗眼部疾病的方法。
-
[EN] BORON-CONTAINING RHO KINASE INHIBITORS<br/>[FR] INHIBITEURS DE LA RHO KINASE CONTENANT DU BORE申请人:PERCIPIAD INC公开号:WO2021011873A1公开(公告)日:2021-01-21The present invention provides boron-containing isoquinoline compounds as protein kinase-modulating compounds. These compounds are useful as neuroprotective and neuro-regenerative agents for the amelioration of glaucoma and other ocular neuropathies.
-
[EN] CERTAIN HETEROCYCLIC SUBSTITUTED IMIDAZO[1,2-A]PYRAZIN-8-YLAMINES AND METHODS OF INHIBITION OF BRUTON'S TYROSINE KINASE BY SUCH COMPOUNDS<br/>[FR] CERTAINES IMIDAZO[1,2-A]PYRAZIN-8-YLAMINES SUBSTITUEES HETEROCYCLIQUES ET METHODES D'INHIBITION DE LA TYROSINE KINASE DE BRUTON UTILISANT CES COMPOSES申请人:CELLULAR GENOMICS INC公开号:WO2005005429A1公开(公告)日:2005-01-20Compounds of formula (I) and all pharmaceutically acceptable forms thereof, are described herein. The variables R1, R2, R3, Z2, and Q, shown in Formula I are defined herein. Pharmaceutical compositions containing one or more compounds of Formula I, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein. Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and/or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and/or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases. Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity. Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with one or more other therapeutic agent. A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.公式(I)的化合物及其所有药物可接受的形态在此描述。公式I中所示的变量R1、R2、R3、Z2和Q在此定义。提供含有公式I的一个或多个化合物,或此类化合物的药物可接受的形态,以及一个或多个药物可接受的载体、辅料或稀释剂的药物组合物。还提供了治疗对抑制酪氨酸激酶活性有反应的某些疾病患者的方法。在某些实施例中,这些疾病对抑制Btk活性和/或B细胞增殖有反应。这些方法包括向患者给药公式I的化合物,以减少疾病的迹象或症状。这些疾病包括癌症、自身免疫和/或炎症性疾病,或急性炎症反应。因此,治疗方法包括给药足够量的本处提供的化合物或盐,以减少这些疾病的症状或减缓这些疾病的进展。其他实施例包括治疗其他动物的方法,包括家畜和驯养的伴侣动物,这些动物患有一种对抑制激酶活性有反应的疾病。治疗方法包括将公式I的化合物作为单一活性剂给药,或将公式I的化合物与一个或多个其他治疗剂组合给药。一种用于确定样本中Btk存在的方法,包括在允许检测Btk活性的条件下将样本与公式I的化合物或其形态接触,检测样本中的Btk活性水平,并据此确定样本中Btk的存在或不存在。
-
[EN] ISOTHIAZOLOQUINOLONES AND RELATED COMPOUNDS AS ANTI-INFECTIVE AGENTS<br/>[FR] ANTI-INFECTIEUX A BASE D'ISOTHIAZOLOQUINOLONES ET DE SELS CORRESPONDANTS申请人:ACHILLION PHARMACEUTICALS INC公开号:WO2005019228A1公开(公告)日:2005-03-03The invention provides compounds and salts of Formula (I) and Formula (II): which possess antimicrobial activity. The invention also provides novel synthetic intermediates useful in making compounds of Formula (I) and Formula (II). The variables A1, R2, R3, R5, R6, R7, A8 and R9 are defined herein. Certain compounds of Formula (I) and Formula (II) disclosed herein are potent and selective inhibitors of bacterial DNA synthesis and bacterial replication. The invention also provides antimicrobial compositions, including pharmaceutical compositions, containing one or more compounds of Formula (I) or Formula (II) and one or more carriers, excipients, or diluents. Such compositions may contain a compound of Formula (I) or Formula (II) as the only active agent or may contain a combination of a compound of Formula (I) or Formula (II) and one or more other active agents. The invention also provides methods for treating microbial infections in animals.本发明提供了具有抗菌活性的公式(I)和公式(II)的化合物及盐类:本发明还提供了用于制造公式(I)和公式(II)化合物的新的合成中间体。变量A1、R2、R3、R5、R6、R7、A8和R9在此文中定义。本文披露的某些公式(I)和公式(II)化合物是细菌DNA合成和细菌复制的强效和选择性抑制剂。本发明还提供了含有一种或多种公式(I)或公式(II)化合物以及一种或多种载体、辅料或稀释剂的抗菌组合物,包括药物组合物。这样的组合物可以只含有公式(I)或公式(II)的化合物作为唯一的活性成分,也可以含有公式(I)或公式(II)的化合物与一种或多种其他活性成分的组合。本发明还提供了用于治疗动物微生物感染的方法。
表征谱图
-
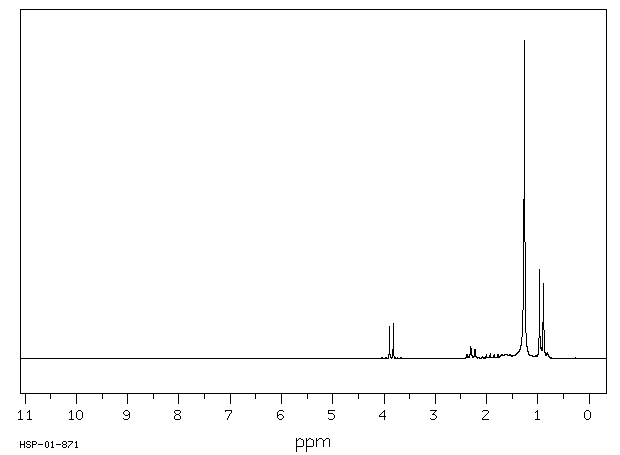
氢谱1HNMR
-
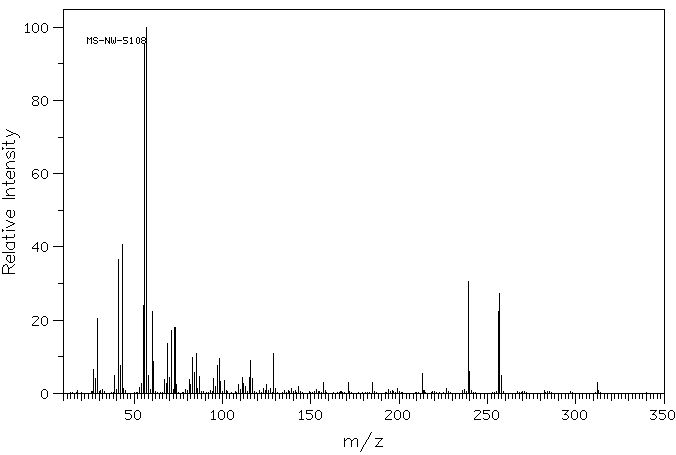
质谱MS
-
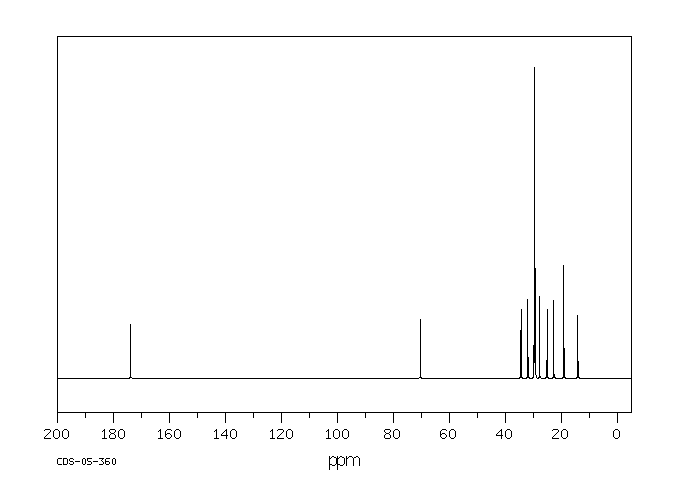
碳谱13CNMR
-
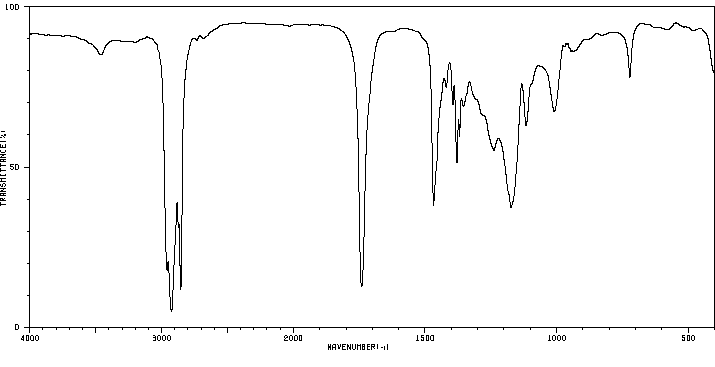
红外IR
-
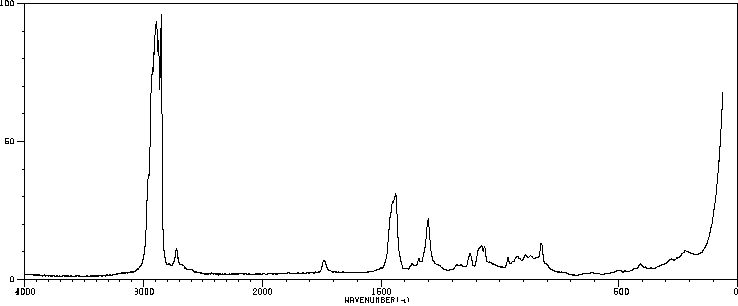
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯