2-苯乙基苯甲酸 | 4890-85-1
中文名称
2-苯乙基苯甲酸
中文别名
Β-苯乙基苯甲酸;2-联苯甲羧酸;邻苯乙基苯甲酸;2-联苄基甲酸
英文名称
o-phenethylbenzoic acid
英文别名
2-phenethylbenzoic acid;2-(2-phenylethyl)benzoic acid;2-phenylethylbenzoic acid;2-bibenzylcarboxylic acid
CAS
4890-85-1
化学式
C15H14O2
mdl
MFCD00002485
分子量
226.275
InChiKey
IOHPVZBSOKLVMN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:127-132 °C
-
沸点:259 °C
-
密度:1.0891 (rough estimate)
-
稳定性/保质期:
避免与不相容材料接触。它会与强氧化剂和强碱发生反应。
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:17
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.133
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2916399090
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | 2-Bibenzylcarboxylic acid Material Safety Data Sheet |
| Synonym: | 2-Phenethylbenzoic acid |
| CAS: | 4890-85-1 |
Synonym:2-Phenethylbenzoic acid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4890-85-1 | 2-Bibenzylcarboxylic acid | 98% | 225-511-8 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled. Can produce delayed pulmonary edema.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4890-85-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white - off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 259 deg C
Freezing/Melting Point: 127-132 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H14O2
Molecular Weight: 226.0968
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4890-85-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Bibenzylcarboxylic acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 4890-85-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4890-85-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4890-85-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
2-苯乙基苯甲酸的用途广泛。它可用作医药合成中间体,如作为神经药物阿米替林(又名阿密替林、依拉维、氨三环庚素等)的重要组成部分。阿米替林是一种重要的临床药物,用于治疗焦虑性或运动性抑郁症、恐慌症、情绪紧张、精神紊乱或肠胃道神经官能症等多种精神类疾病。
2-苯乙基苯甲酸的制备过程如下:向室温下的适量双组份有机溶剂(体积比1:3的1,4-二氧六环与乙腈的混合物)中加入100mmol上式(I)化合物、100mmol上式(II)化合物、8mmol催化剂二(氰基甲基)二氯化钯、10mmol氮多齿配体、180mmol氧化剂双(三氟乙酸)碘苯和200mmol有机碱二甲氨基吡啶,升温至70℃并在该温度下搅拌反应24小时。反应结束后,趁热过滤,将滤液自然冷却至室温,调节pH值为中性,再用足量饱和碳酸钠水溶液洗涤2-4次,合并有机相,降温至2-6℃,析出固体,抽滤并用无水乙醇充分洗涤,真空干燥,从而得到白色晶体状的上式(III)化合物2-苯乙基苯甲酸,产率为97.2%,HPLC分析得知其纯度为98.7%。
2-苯乙基苯甲酸具有以下化学性质:固体。熔点130-132℃,沸点259℃。
该化合物主要用作医药阿米替林的中间体。
生产方法如下: (1)缩合:苯酐与苯乙酸缩合得苄叉酞酐。 (2)水解、加氢、酸中和:苄叉酞酐水解为邻苯乙酰苯甲酸钠,再加氢得邻β-苯乙基苯甲酸钠,用盐酸中和,经乙醇精制而得邻β-苯乙基苯甲酸。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 2-(2-phenylethyl)benzoate 194605-53-3 C16H16O2 240.302 —— ethyl 2-phenethylbenzoate 5505-02-2 C17H18O2 254.329 2-(苯基乙酰基)苯甲酸 2-(2-phenylacetyl)benzoic acid 33148-55-9 C15H12O3 240.258 —— 2-(2-Bromethyl)-benzoesaeure 42247-76-7 C9H9BrO2 229.073 2-苯乙基苯甲醛 2-phenethylbenzaldehyde 32832-96-5 C15H14O 210.276 对甲苯甲酸 ortho-methylbenzoic acid 118-90-1 C8H8O2 136.15 3-苄基氯苯酞 3-benzyl-3H-isobenzofuran-1-one 7011-98-5 C15H12O2 224.259 —— 3-phenyl-isochroman-1-one 146518-60-7 C15H12O2 224.259 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— [2-(4-bromophenyl)ethyl]benzoic acid 3973-52-2 C15H13BrO2 305.171 —— methyl 2-(2-phenylethyl)benzoate 194605-53-3 C16H16O2 240.302 2-苯乙基苯甲醇 2-phenethylbenzyl alcohol 835-78-9 C15H16O 212.291 2-苯乙基苯甲醛 2-phenethylbenzaldehyde 32832-96-5 C15H14O 210.276 —— (β-Dimethylaminoethyl)-2-(2-phenylethyl)benzoat 15150-08-0 C19H23NO2 297.397 —— (3-Dimethyl-aminopropyl)-2-(2-phenylethyl)-benzoat 14188-00-2 C20H25NO2 311.424 —— 3-Phenaethyl-phthalsaeure 35157-38-1 C16H14O4 270.285 —— bibenzyl-2-carboxamide 36795-31-0 C15H15NO 225.29 2-(2-苯基乙基)苯甲酰氯 diphenylethane-2-carboxylic acid chloride 36795-27-4 C15H13ClO 244.721 1-甲基-2-(2-苯基乙基)苯 1-methyl-2-phenethylbenzene 34403-05-9 C15H16 196.292 3-苄基氯苯酞 3-benzyl-3H-isobenzofuran-1-one 7011-98-5 C15H12O2 224.259 —— 3-phenyl-isochroman-1-one 146518-60-7 C15H12O2 224.259 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:合成2'-乙酰氨基-2,3; 6,7-二苯并噻吩并2-乙基乙酰氨基-9,9-二甲基氟摘要:Es wird die Herstellung von 2'-Acetamino-2,3; 6,7-二苯并噻吩基(X)和2-乙酰氨基-9,9-二甲基芴苯甲酸。X wurde aus 2,3; (II)überdas 2',6“ -dibenzo-cyclohepta-2,6-dien-1-on,2” -Dinitro-2,3; 6,7-二苯并肌钙蛋白(V)的合成。DOI:10.1002/hlca.19530360635
-
作为产物:参考文献:名称:金属三氟甲磺酸酯促进内酯氢解成羧酸的综合研究:从合成和机理的角度摘要:内酯直接氢解为羧酸(即不接触羰基的C烷氧基-O键的氢解)通常是困难的,因为当前使用布朗斯台德酸作为催化剂的策略通常需要苛刻的条件,例如高温和高温H 2压力。在此,我们报告了已开发的无溶剂催化转化方法,其中W(OTf)6被认为可以促进氢解过程。该策略可以在特别温和的条件下(例如,反应温度<150°C和1 atm H 2的条件下)有效地将内酯氢化为羧酸),并显示出较宽的基材范围。另外,该催化方案可以进一步应用于作为可再生聚合物的聚羟基链烷酸酯的氢解成相应的直链羧酸。随后进行了广泛的机理研究,密度泛函理论计算揭示了一种反应模式,包括在W(OTf)6的帮助下C═O键的完全裂解催化剂。此外,通过电喷雾电离质谱已成功检测到该机理中产生的关键中间体,即具有OTf部分的moiety。通过与布朗斯台德酸催化体系的比较,研究证实了OTf部分的存在可以显着降低与重排和消除过程相关的障碍。同时,重点放在阴离子发挥DOI:10.1021/acscatal.7b01569
文献信息
-
[EN] NOVEL COMPOUNDS, THEIR PREPARATION AND USE<br/>[FR] NOUVEAUX COMPOSES, LEUR PREPARATION ET LEUR UTILISATION申请人:NOVO NORDISK AS公开号:WO2005105736A1公开(公告)日:2005-11-10Novel compounds of the general formula (I), the use of these compounds as phar- maceutical compositions, pharmaceutical compositions comprising the compounds and methods of treatment employing these compounds and compositions. The present compounds may be useful in the treatment and/or prevention of conditions mediated by Peroxisome Proliferator-Activated Receptors (PPAR), in particular the PPARδ suptype.
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS AND USES THEREOF申请人:Florjancic S. Alan公开号:US20080058335A1公开(公告)日:2008-03-06The present invention relates to compounds of formula (I), or pharmaceutical salts, prodrugs, salts of prodrugs, or combinations thereof, wherein R 1 , R 2 , R 3 , and L 1 are defined in the specfication, compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions. The present invention also relates to compounds of formula (II), or pharmaceutical salts, prodrugs, salts of prodrugs, or combinations thereof, wherein R 1a , R 2a and (Rx)n are as defined in the specification, compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions.本发明涉及式(I)的化合物,或药用盐、前药、前药的盐或其组合物, 其中R 1 ,R 2 ,R 3 和L 1 在规范中定义,包含这种化合物的组合物,以及使用这种化合物和组合物治疗疾病和疾病的方法。本发明还涉及式(II)的化合物,或药用盐、前药、前药的盐或其组合物, 其中R 1a ,R 2a 和(Rx)n如规范中定义,包含这种化合物的组合物,以及使用这种化合物和组合物治疗疾病和疾病的方法。
-
Scalable Electrochemical Dehydrogenative Lactonization of C(sp<sup>2</sup>/sp<sup>3</sup>)–H Bonds作者:Sheng Zhang、Lijun Li、Huiqiao Wang、Qian Li、Wenmin Liu、Kun Xu、Chengchu ZengDOI:10.1021/acs.orglett.7b03617日期:2018.1.5A practical, electrochemical method is developed for the direct dehydrogenative lactonization of C(sp2/sp3)–H bonds under external oxidant- and metal-free conditions, delivering diverse lactones, including coumarin derivatives with excellent regioselectivity. The scalable nature of this newly developed electrochemical process was demonstrated on a 40 g scale following an operationally simple protocol
-
Inhibitors of HPV E1 helicase enzyme申请人:——公开号:US20020128262A1公开(公告)日:2002-09-12The invention is concerned with novel benzodiazepine derivatives, a process for their manufacture, pharmaceutical compositions and the use of such compounds in medicine. In particular, the compounds are inhibitors of the human papillomavirus E1 helicase enzyme which is involved in viral replication. Consequently the compounds of this invention may be advantageously used as therapeutic agents for HPV mediateddiseases.
-
Branched chain amino acid-dependent aminotransferase inhibitors and their use in the treatment of neurodegenerative diseases申请人:——公开号:US20030149110A1公开(公告)日:2003-08-07The invention relates to BCAT inhibitors and the use thereof for treating or preventing neuronal loss associated with stroke, ischemia, CNS trauma, hypoglycemia and surgery, as well as treating neurodegenerative diseases including Alzheimer's disease, amyotrophic lateral sclerosis, Huntington's disease and Down's syndrome, treating or preventing the adverse consequences of the overstimulation of the excitatory amino acids, treating anxiety, psychosis, convulsions, aminoglycoside antibiotics-induced hearing loss, migraine headache, chronic pain, neuropathic pain, Parkinson's disease, diabetic retinopathy, glaucoma, CMV retinitis, urinary incontinence, opioid tolerance or withdrawal, and inducing anesthesia, as well as for enhancing cognition.
表征谱图
-
氢谱1HNMR
-
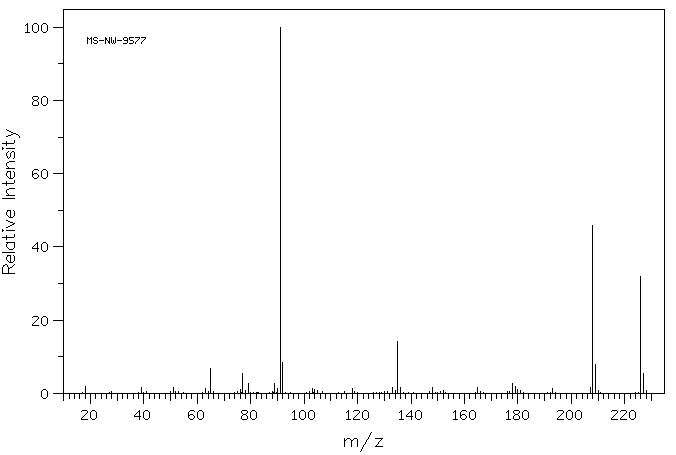
质谱MS
-
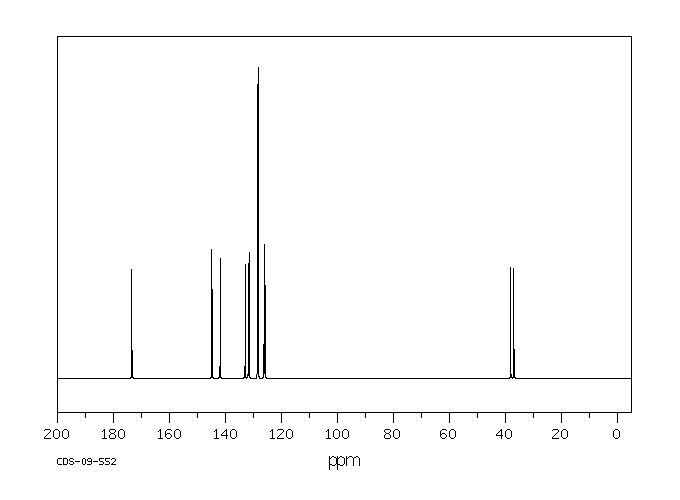
碳谱13CNMR
-
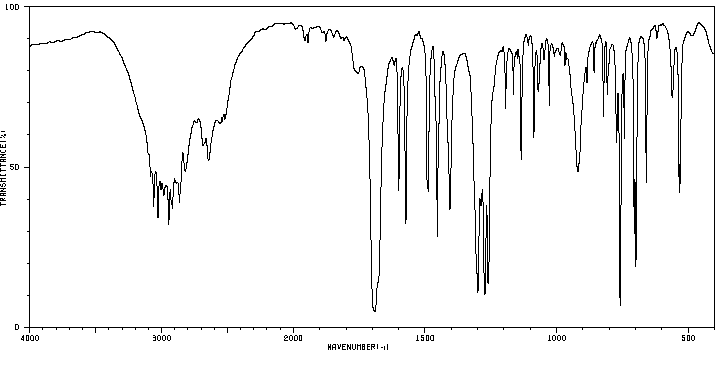
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯