(3E,5E)-3,5-庚二烯-2-酮 | 18402-90-9
中文名称
(3E,5E)-3,5-庚二烯-2-酮
中文别名
3,5-庚二烯-2-酮,(3E,5E)-(9CI)
英文名称
(3E,5E)-3.5-heptadien-2-one
英文别名
(3E,5E)-hepta-3,5-dien-2-one;2,4-Heptadien-6-one
CAS
18402-90-9
化学式
C7H10O
mdl
——
分子量
110.156
InChiKey
SWGLACWOVFCDQS-VNKDHWASSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:65-67 °C(Press: 12 Torr)
-
密度:0.857±0.06 g/cm3(Predicted)
-
LogP:1.260 (est)
-
保留指数:1095
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:参考文献:名称:甲硅烷基缩醛三烯的分子内狄尔斯-阿尔德反应。烯丙基取代的立体选择性的影响摘要:描述了在二烯或亲二烯体的烯丙基位置具有甲基的甲硅烷基乙缩醛三烯的合成和分子内热Diels-Alder反应。提出了一个模型来说明观察到的选择性。通过衍生物的X射线冷冻图谱分析明确地确定了环加合物的结构。DOI:10.1016/0040-4039(92)88103-c
-
作为产物:描述:参考文献:名称:Ley, Steven V.; Cox, Liam R.; Meek, Graham, Journal of the Chemical Society. Perkin transactions I, 1997, # 22, p. 3299 - 3313摘要:DOI:
文献信息
-
Retinol analogues. Part I. Synthesis of acyclic analogues of retinoic acid作者:O. P. H. Augustyn、P. de Wet、C. F. Garbers、L. C. F. Lourens、E. Neuland、D. F. Schneider、K. SteynDOI:10.1039/j39710001878日期:——Various C19 analogues of retinoic acid have been prepared from isopropyl t-butyl ketone. Potassium hydrogen sulphate in dimethyl sulphoxide proved an effective reagent for the elimination of water from intermediate hydroxyderivatives, but dehydration of 9-hydroxy-6,10-dimethyl-9-t-butylundeca-3,5,7-trien-2-one yielded 2-methyl-5-(4-isopropyl-1,5,5-trimethylhexa-1,3-dienyl)furan and 9-isopropyl-6,9
-
Cyanotrimethylsilane as a versatile reagent for introducing cyanide functionality作者:Kiitiro Utimoto、Yukio Wakabayashi、Takafumi Horiie、Masaharu Inoue、Yuho Shishiyama、Michio Obayashi、Hitosi NozakiDOI:10.1016/s0040-4020(01)88595-1日期:1983.1Cyanotrimethylsilane adds to some ⇌,β-unsaturated ketones in conjugate manner under the catalytic action of Lewis acids such as triethylaluminium, aluminium chloride, and SnCl2. Hydrolysis of the products gives β-cyano ketones which are identical to the hydrocyanated products of the starting enones. The title silicon reagent reacts with acetals and orthoesters under the catalytic action of SnCI2 or BF3-OEt2
-
RUTHENIUM COMPLEX, PROCESS FOR PRODUCING THE SAME AND PROCESS FOR PRODUCING THIN FILM申请人:TOSOH CORPORATION公开号:US20030088116A1公开(公告)日:2003-05-08A ruthenium-containing thin film is produced by the chemical vapor deposition method etc. with the use of an organometallic ruthenium compound represented by the general formula (1), specific example of which is (2,4-dimethyl-pentadienyl)(ethylcyclopentadienyl) ruthenium: 1 or an organometallic ruthenium compound represented by the general formula (7), specific example of which is carbonylbis(2-methyl-1,3-pentadiene) ruthenium: 2 as the precursor.
-
Aldolisation-type reaction versus Michael-type addition. Hemiacetal vinylogs: Versatile synthons.作者:Pierre Duhamel、Jérôme Guillemont、Jean-Marie Poirier、Pierre ChabardesDOI:10.1016/s0040-4039(00)60526-9日期:1993.6Depending on the steric hindrance of the reaction centers, hemiacetal vinylogs 1 in the presence of BF3.OEt2 can, with enol ethers, lead to a Michael-type addition (4) or an aldolisation-type reaction (3). Hemiacetal vinylog 1b always yields the second reaction leading to β-methoxy-γ,δ-ethylenic carbonyl compounds 4 precursors of polyenic carbonyl compounds 5. Crotonaldehyde and methanol can be used
-
Diastereoselective addition reactions to carbonyl groups in the side-chain of π-allytricarbonyliron lactone complexes
表征谱图
-
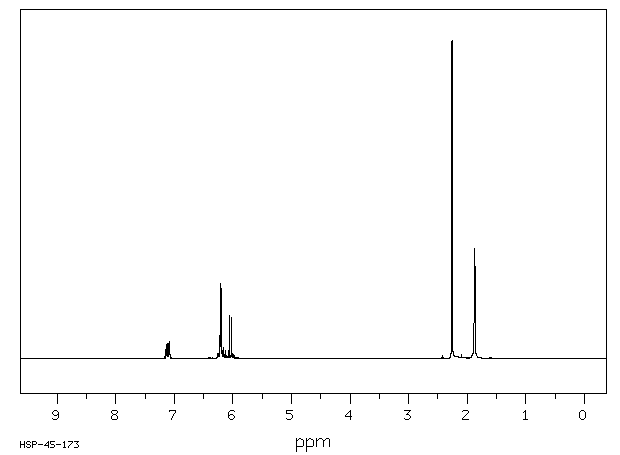
氢谱1HNMR
-
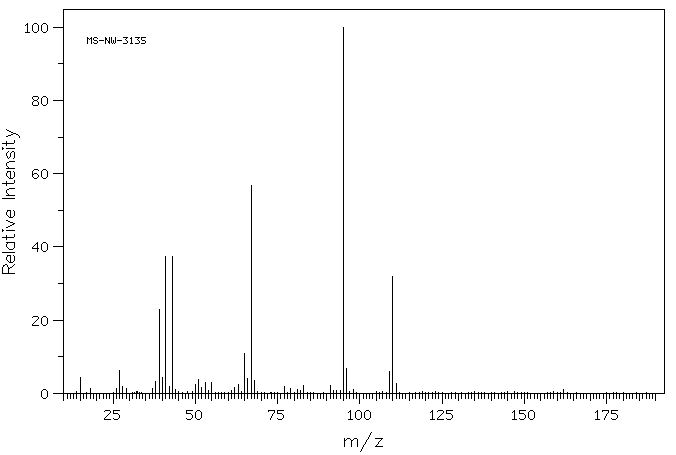
质谱MS
-
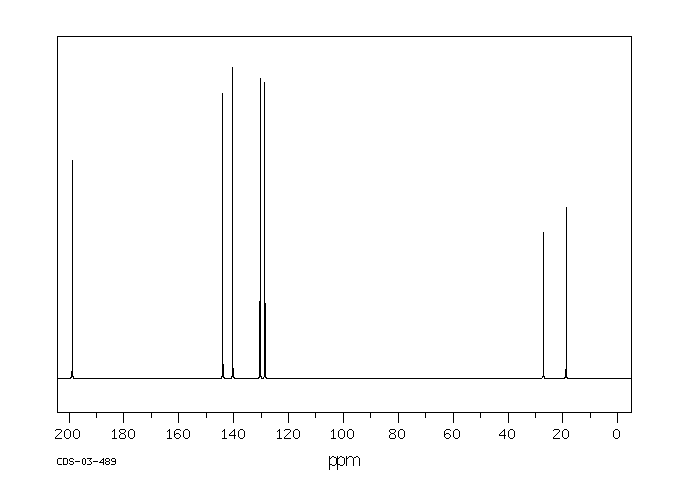
碳谱13CNMR
-
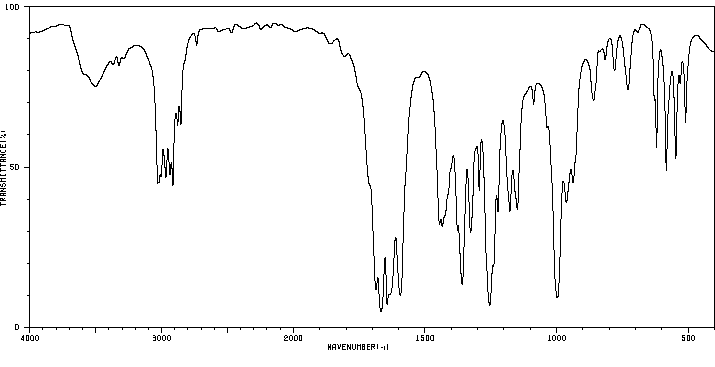
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷