1,3-二乙酰基吲哚 | 17537-64-3
中文名称
1,3-二乙酰基吲哚
中文别名
——
英文名称
1,3-diacetyl-1H-indole
英文别名
1,3-diacetylindole;1,1'-(1H-indole-1,3-diyl)diethanone;1,1’-(1H-indole-1,3-diyl)bis(ethan-1-one);1-(1-acetylindol-3-yl)ethanone
CAS
17537-64-3
化学式
C12H11NO2
mdl
MFCD00039692
分子量
201.225
InChiKey
STUZJORZRZCLRI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:144-147°C
-
沸点:338.8±15.0 °C(Predicted)
-
密度:1.16±0.1 g/cm3(Predicted)
-
保留指数:1903
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:39.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
安全说明:S22,S24/25
-
海关编码:2933990090
-
危险品标志:Xi
-
危险性防范说明:P264,P270,P301+P312,P330
-
危险性描述:H302
-
储存条件:常温密闭避光、通风干燥的惰性气体环境中保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 1,3-Diacetylindole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1,3-Diacetylindole
CAS number: 17537-64-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C12 H11 NO2
Molecular weight: 201.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 1,3-Diacetylindole
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 1,3-Diacetylindole
CAS number: 17537-64-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C12 H11 NO2
Molecular weight: 201.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 1-acetyl-3-ethylindole 88636-48-0 C12H13NO 187.241 3-乙酰吲哚 1-(1H-indol-3-yl)-ethanone 703-80-0 C10H9NO 159.188 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3-diacetyl-2-[2-(triethoxysilyl)ethyl]indole 1233131-36-6 C20H29NO5Si 391.539 3-乙酰吲哚 1-(1H-indol-3-yl)-ethanone 703-80-0 C10H9NO 159.188 1-(6-硝基-1H-吲哚-3-基)乙酮 3-acetyl-6-nitroindole 4993-92-4 C10H8N2O3 204.185 1-(5-硝基-1H-吲哚-3-基)乙酮 3-acetyl-5-nitroindole 4771-10-2 C10H8N2O3 204.185
反应信息
-
作为反应物:描述:参考文献:名称:三组分偶联-氧化酰胺化-杂环环化:吲哚生物碱Hamacanthin A和反式-2,5-双(3'-吲哚基)哌嗪的合成摘要:描述了抗真菌海洋双(吲哚)生物碱、hamacanthin A 和反式-2,5-双(3'-吲哚基)哌嗪的简洁和高度收敛的合成。hamacanthin A 的全合成是通过 2,2-dibromo-1-(1H-indol-3-yl)ethanone 与 1-(1H-indol-3-yl)ethane-1 的氧化酰胺化-化学选择性杂环环化完成的, 2-二胺。用硼氢化铝还原 desbromo hamacanthin 得到生物碱反式-2,5-双(3'-吲哚基)哌嗪。还开发了两种新颖且方便的合成吲哚基-1,2-二氨基乙烷的方案,产率中等至良好。DOI:10.1055/s-0036-1588961
-
作为产物:描述:1-acetyl-3-ethylindole 在 亚硝酸特丁酯 、 N-羟基邻苯二甲酰亚胺 、 氧气 作用下, 以 乙腈 为溶剂, 80.0 ℃ 、101.33 kPa 条件下, 反应 24.0h, 以71%的产率得到1,3-二乙酰基吲哚参考文献:名称:克服了烷基吡啶和相关烷基杂芳烃的有机催化好氧氧化成酮的电子吸收和产物抑制作用。摘要:已经开发了烷基和芳基杂环的有机催化的需氧苄基CH氧化。这种无过渡金属的方法能够克服杂苄基自由基氧化中的吸电子作用以及产物抑制作用。可以在相对温和的条件下以中等至高产率制备各种带有N-杂环基的酮。DOI:10.1021/acs.joc.9b03205
文献信息
-
Efficient and simple methods for the introduction of the sulfonyl, acyl and alkyl protecting groups on the nitrogen of indole and its derivatives作者:Olívia Ottoni、Rosimeire Cruz、Robledo AlvesDOI:10.1016/s0040-4020(98)00865-5日期:1998.11The benzenesulfonylation, acetylation, methylation and benzylation at the 1 position of indole and its derivatives with bases such as NaOH, KOH and NEt3 are presented. By using weaker bases than the traditional ones (BuLi, NaNH2, etc.), the process is simpler, more general and leads to the products in 80–100% yields. The chemoselectivity in compounds bearing more than one nitrogen is also demonstrated
-
Regioselective Hydroarylation Reactions of C3 Electrophilic<i>N</i>-Acetylindoles Activated by FeCl<sub>3</sub>: An Entry to 3-(Hetero)arylindolines作者:Rodolphe Beaud、Régis Guillot、Cyrille Kouklovsky、Guillaume VincentDOI:10.1002/chem.201400284日期:2014.6.10indole nucleus to generate a quaternary center at C3 and leads regioselectively to 3‐arylindolines. Optimization, scope (50 examples), practicability (gram scale, air atmosphere, room temperature), and mechanistic insights of this process are presented. Synthetic transformations of the indoline products into drug‐like compounds are also described.
-
The acetylation of 3-acylindoles.作者:TOHRU HINO、YASUHIRO TORISAWA、MASAKO NAKAGAWADOI:10.1248/cpb.30.2349日期:——Acetylation of 3-acetylindole (9) with acetyl chloride-aluminum chloride in nitrobenzene or methylene chloride gave 3, 5-diacetylindole (10) as the major product, together with 3, 6- and 3, 7-diacetylindole (11, 12) as minor products. The similar acetylation of ethyl 3-indolecarboxylate (16) also gave the 5-acetyl derivative (17a) as the major product, together with the 6-and 7-acetyl derivatives (18a, 19) as minor products. Oxidation of 1, 3-diacetylindole (15) with lead tetratrifluoroacetate gave 3-indolinones (22, 23) and a dimeric product (24).
-
Hydrogen bond donor solvents enabled metal and halogen-free Friedel–Crafts acylations with virtually no waste stream作者:Guangchang Liu、Bo XuDOI:10.1016/j.tetlet.2018.01.026日期:2018.3halogen-free Friedel–Crafts acylation protocol with virtually no waste stream generation. We propose a hydrogen bonding donor solvent will form a hydrogen bonding network and may provide significant rate enhancement for Friedel–Crafts reactions. Trifluoroacetic acid is one of the strongest H-bond donor solvents, which is also volatile and can be easily recovered by distillation without need for reaction workup
-
A New Strategy toward Indole Alkaloids Involving an Intramolecular Cycloaddition/Rearrangement Cascade作者:Albert Padwa、Michael A. Brodney、Stephen M. Lynch、Paitoon Rashatasakhon、Qiu Wang、Hongjun ZhangDOI:10.1021/jo049808i日期:2004.5.1The intramolecular Diels−Alder reaction between an amidofuran moiety tethered onto an indole component was examined as a strategy for the synthesis of Aspidosperma alkaloids. Furanyl carbamate 23 was acylated using the mixed anhydride 26 to provide amidofuran 22 in 68% yield. Further N-acylation of this indole furnished 27 in 88% yield. Cyclization precursors were prepared by removing the carbamate拴在吲哚成分上的酰胺基呋喃部分之间的分子内Diels-Alder反应已被检测为合成Asperdosperma生物碱的策略。使用混合酸酐26将氨基甲酸呋喃酯23酰化,以68%的产率提供酰胺基呋喃22。该吲哚的进一步N-酰化得到27,收率88%。环化前体通过除去氨基甲酸酯部分,随后加入N-制备-用适当的卤代烷进行烷基化。酰胺基氮原子上的较大取代基会导致酰胺基呋喃的反应性s-反式构型更高,从而促进Diels-Alder环加成反应。该反应需要在吲哚氮上存在吸电子取代基,以便进行环加成反应。的治疗N-烯丙基bromoenamide 48与Ñ -Bu 3 SNH / AIBN优先引导到6-内TRIG环化产物50,具有较高的稀释条件下而获得的最好的产率(91%)。最初衍生自48的环己烯基产生五环杂环50 通过直接的6-endo trig环化,或通过乙烯基自由基重排途径。
表征谱图
-
氢谱1HNMR
-
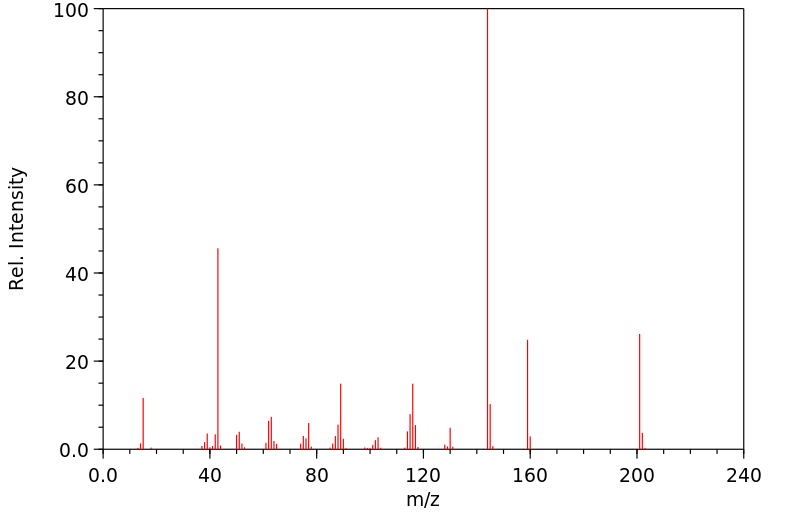
质谱MS
-
碳谱13CNMR
-
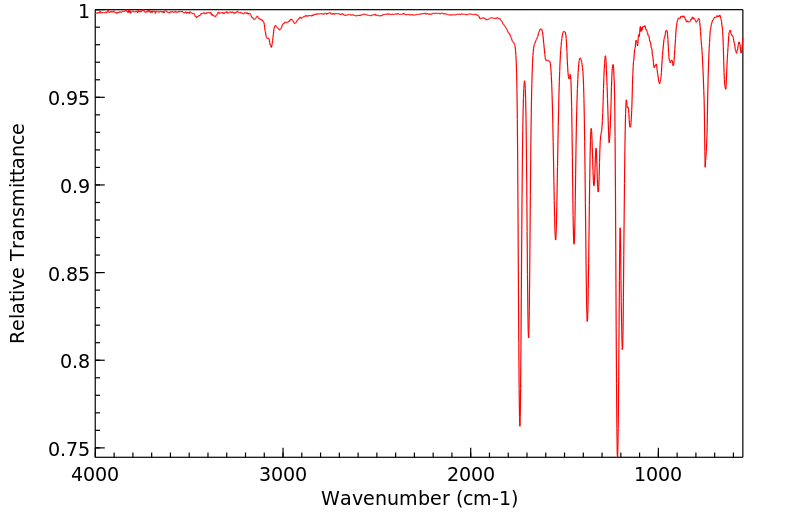
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3