7-methylenenorbornadiene | 37846-63-2
中文名称
——
中文别名
——
英文名称
7-methylenenorbornadiene
英文别名
7-Methylennorbornadien;7-Methylen-bicyclo<2.2.1>hepta-2,5-dien;Methylenbicyclo<2.2.1>heptadien;7-Methylen-norbornadien;Methylen-norbornadien;Methylenorbornadien;Bicyclo(2.2.1)hepta-2,5-diene, 7-methylene-;7-methylidenebicyclo[2.2.1]hepta-2,5-diene
CAS
37846-63-2
化学式
C8H8
mdl
——
分子量
104.152
InChiKey
OWNNLXWCBCZDHM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:8
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:HOFFMANN, R. W.;RIEMANN, A.;MAYER, B., CHEM. BER., 1985, 118, N 6, 2493-2513摘要:DOI:
-
作为产物:描述:3-methylenetetracyclo<3.2.0.02,7,04,6>heptane 在 Rh catalyst 作用下, 以 四氯化碳 为溶剂, 生成 7-methylenenorbornadiene参考文献:名称:Unsaturated heterocyclic systems. XC. Uniparticulate electrophilic addition as a probe of possible bicycloaromatic and antibicycloaromatic carbonium ion character. Reactions of chlorosulfonyl isocyanate with exocyclic methylene precursors to such cations摘要:DOI:10.1021/jo00950a024
文献信息
-
Bicyclofulvene—VI作者:Reinhard W. Hoffman、Johannes BechererDOI:10.1016/0040-4020(78)80143-4日期:1978.1Ketene imminium ion (4), diphenyl ketene, tosyl isocyanate and tetracyanoethylene add selectively to the semicyclic double bond of 1. This is interpreted to result from the polarity of the semicyclic double bond in 1.
-
Synthesis of tetracyclo[3.3.0.02.4.03.6]oct-7-ene and some of its derivatives作者:J. Stapersma、I.D.C. Rood、G.W. KlumppDOI:10.1016/0040-4020(82)85065-5日期:1982.1A full account is given of the synthesis of the title compound, its 7,8-dihydro-analogue and of some of its derivatives.完整说明了标题化合物,其7,8-二氢类似物及其某些衍生物的合成。
-
An unusual bilaterally flanked olefin; X-ray crystal structure of 5,14;7,12-bis-(o-benzeno)-6,13-ethenylidene-5,5a,6,6a,7,12,12a,13,13a,14-decahydropentacene作者:Douglas N. Butler、Indranil Gupta、Winnie Wong Ng、Stanley C. NyburgDOI:10.1039/c39800000596日期:——Spectroscopic and chemical evidence reveals the intramolecular π–π orbital proximity in the novel Diels–Alder adducts (7) and (9); the X-ray crystal structure of (9) is reported.光谱和化学证据揭示了新型狄尔斯-阿尔德加合物(7)和(9)中分子内的π - π轨道接近性。报告了(9)的X射线晶体结构。
-
Hoffmann, Reinhard W.; Riemann, Achim; Mayer, Bernhard, Chemische Berichte, 1985, vol. 118, # 6, p. 2493 - 2513作者:Hoffmann, Reinhard W.、Riemann, Achim、Mayer, BernhardDOI:——日期:——
-
The Putative Role of Homoconjugation in Radical Cations: Electron Transfer Photochemistry of 7-Methylenenorbornadiene and 7-Methylenequadricyclane作者:Hengxin Weng、Xue-Mei Du、Heinz D. RothDOI:10.1021/ja00106a017日期:1995.1Photoinduced electron transfer from 7-methylenenorbornadiene, MN, and 7-methylenequadricyclane, MQ, to an excited sensitizer/acceptor in the presence of methanol generates products of several structure types. All products require nucleophilic capture of the radical cations, MN(.+) and MQ(.+), by methanol, followed by rapid rearrangements of the resulting free radicals to C-. as the key intermediate. The short lifetime of the primary products of capture is ascribed to the allylic nature of their C-4-C-5 bonds. The stereochemistry of the methoxy groups in the products indicates that the nucleophile attacks MQ(.+) exclusively from the ''exo'' face (''backside'' attack), whereas MN(.+) shows, in addition, a limited degree of attack from the ''endo'' face.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
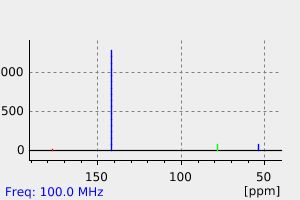
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-