三月桂胺 | 102-87-4
中文名称
三月桂胺
中文别名
三正十二胺;三-n-十二基胺;三(十二烷基)叔胺;氢离子载体,I;N,N-双十二烷基-1-十二胺;三-十二烷基叔胺;TA12
英文名称
tridodecylamine
英文别名
trilaurylamine;tri(n-dodecyl)-amine;tris(dodecyl)amine;Tri-n-laurylamin;N,N-didodecyldodecan-1-amine
CAS
102-87-4
化学式
C36H75N
mdl
MFCD00008971
分子量
521.998
InChiKey
SWZDQOUHBYYPJD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:16 °C
-
沸点:220-228 °C/0.03 mmHg (lit.)
-
密度:0.823 g/mL at 25 °C (lit.)
-
蒸气密度:>1 (vs air)
-
闪点:>230 °F
-
LogP:16.932 (est)
-
物理描述:Liquid
-
稳定性/保质期:
避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):17
-
重原子数:37
-
可旋转键数:33
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:2921199090
-
储存条件:储存于阴凉、通风的库房。远离火种、热源,并与氧化剂分开存放,严禁混合储存。需配备相应的消防器材。库区应配置泄漏应急处理设备和适合的收集材料。
SDS
1.1 产品标识符
: 氢离子载体 I
产品名称
1.2 鉴别的其他方法
Hydrogen ionophore I
TridodECylamine
Proton ionophore I
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Hydrogen ionophore I
别名
TridodECylamine
Proton ionophore I
: C36H75N
分子式
: 521.99 g/mol
分子量
组分 浓度或浓度范围
TridodECylamine
-
CAS 号 102-87-4
EC-编号 203-063-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用惰性吸附材料吸收并当作危险废品处理。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
充气保存 对空气敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
沉浸保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.4 mm
溶剂渗透时间: > 480 min
测试过的物质Camatril® ( Z677442, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 30 min
测试过的物质Dermatril® ( Z677272, 规格 M)
0, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不 同于EN
374规定的条件下应用,请与EC批准的手套的供应 商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
220 - 228 °C 在 0.04 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
18.02 - (空气= 1。0)
m) 相对密度
0.823 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
对空气敏感。
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 氢离子载体 I
产品名称
1.2 鉴别的其他方法
Hydrogen ionophore I
TridodECylamine
Proton ionophore I
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。 如仍觉眼睛刺激:求医/就诊.
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Hydrogen ionophore I
别名
TridodECylamine
Proton ionophore I
: C36H75N
分子式
: 521.99 g/mol
分子量
组分 浓度或浓度范围
TridodECylamine
-
CAS 号 102-87-4
EC-编号 203-063-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
用惰性吸附材料吸收并当作危险废品处理。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
充气保存 对空气敏感。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
沉浸保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.4 mm
溶剂渗透时间: > 480 min
测试过的物质Camatril® ( Z677442, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: > 30 min
测试过的物质Dermatril® ( Z677272, 规格 M)
0, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不 同于EN
374规定的条件下应用,请与EC批准的手套的供应 商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
220 - 228 °C 在 0.04 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
18.02 - (空气= 1。0)
m) 相对密度
0.823 g/mL 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
对空气敏感。
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
用途:用于提取稀土元素的萃取剂
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 双十二烷基胺 N,N-didodecylamine 3007-31-6 C24H51N 353.676 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二十二烷基甲酰胺 N,N-didodecylformamide 55282-35-4 C25H51NO 381.686
反应信息
-
作为反应物:描述:参考文献:名称:在 NH3 水溶液中用 1,3-Diiodo-5,5-二甲基乙内酰脲氧化转化伯醇和伯胺、仲胺和叔胺为相应的腈类摘要:各种伯醇和伯胺、仲胺和叔胺在 60° 的氨水 (NH 3 ) 中通过 l,3-二碘-5,5-二甲基乙内酰脲 (DIH) 以良好的收率氧化并有效地转化为相应的腈C。DOI:10.1055/s-2007-967954
-
作为产物:描述:十二腈 在 aluminum oxide 、 copper-chromium powder 甲醇 、 氢气 作用下, 249.9 ℃ 、5.0 MPa 条件下, 反应 25.0h, 以5%的产率得到三月桂胺参考文献:名称:Selectivity control in substituted fatty amines synthesis from esters or nitriles in the presence of bifunctional catalysts摘要:摘要 以氧化铝或石墨为载体并以钡为促进剂的铜铬酸盐型催化剂被用于从酯、酸或腈与氨、甲醇和氢一步合成叔脂肪胺[R2NCH3 或 RN(CH3)2]。通过 X 射线光电子能谱和吸附实验对催化剂的表面组成进行了研究,结果表明,催化剂的选择性与是否存在用钡稳定的分散良好的 CuCrO2 相有关。此外,还对影响铜催化剂稳定性的元素进行了研究,发现促进剂或/和载体增加了铜的表面积,并提高了催化剂在水或氨存在下的稳定性。DOI:10.1007/bf02540543
-
作为试剂:描述:gallium(III) trichloride 、 三甲基铝 在 三月桂胺 作用下, 以 5,5-dimethyl-1,3-cyclohexadiene 为溶剂, 反应 1.0h, 以93.3%的产率得到三甲基镓参考文献:名称:トリアルキルガリウムの製造方法摘要:【问题】提供一种制造三烷基镓的简便方法,使后处理不复杂。 【解决方法】在存在式(3)所示的胺化合物和四级铵盐中的至少一种的情况下,将三卤化镓和三烷基铝反应,制备式(4)所示的三烷基镓。(其中,R1是直链或支链烷基,其碳数为C7-12;R是C1-6的烷基。)【选择图】无公开号:JP2016029026A
文献信息
-
USE OF NITROGEN COMPOUNDS QUATERNISED WITH ALKYLENE OXIDE AND HYDROCARBYL-SUBSTITUTED POLYCARBOXYLIC ACID AS ADDITIVES IN FUELS AND LUBRICANTS申请人:BASF SE公开号:US20160130514A1公开(公告)日:2016-05-12The invention relates to the use of quaternized nitrogen compounds as a fuel and lubricant additive or kerosene additive, such as in particular as a detergent additive, for decreasing or preventing deposits in the injection systems of direct-injection diesel engines, in particular in common rail injection systems, for decreasing the fuel consumption of direct-injection diesel engines, in particular of diesel engines having common rail injection systems, and for minimizing the power loss in direct-injection diesel engines, in particular in diesel engines having common rail injection systems; the invention further relates to the use as an additive for petrol, in particular for operation of DISI engines.该发明涉及将季铵化氮化合物用作燃料和润滑剂添加剂或煤油添加剂,特别是作为清洁剂添加剂,用于减少或预防直喷柴油发动机的喷射系统中的沉积物,在特定是在共轨喷射系统中,用于降低直喷柴油发动机的燃料消耗,特别是具有共轨喷射系统的柴油发动机,并用于减少直喷柴油发动机的功率损失,特别是在具有共轨喷射系统的柴油发动机中;该发明还涉及将其用作汽油添加剂,特别是用于DISI发动机的运行。
-
PROCESS FOR PREPARING FORMIC ACID申请人:Fachinetti Giuseppe公开号:US20130006015A1公开(公告)日:2013-01-03A process for preparing formic acid by hydrogenation of carbon dioxide in the presence of a tertiary amine (I) and a catalyst at a pressure of from 0.2 to 30 MPa abs and a temperature of from 20 to 200° C., wherein the catalyst is a heterogeneous catalyst comprising gold.
-
一种含烷基和芳基嘧啶类化合物的合成方法申请人:新乡医学院公开号:CN111362880B公开(公告)日:2023-01-13
-
FLUORINATED SULFONATE ESTERS OF ARYL KETONES FOR NON-IONIC PHOTO-ACID GENERATORS申请人:International Business Machines Corporation公开号:US20180046077A1公开(公告)日:2018-02-15Non-ionic photo-acid generating (PAG) compounds were prepared that contain an aryl ketone group having a perfluorinated substituent alpha to the ketone carbonyl. The non-polymeric PAGs release a sulfonic acid when exposed to high energy radiation such as deep UV or extreme UV light. The photo-generated sulfonic acid has a low diffusion rate in an exposed resist layer subjected to a post-exposure bake (PEB) at 100° C. to 150° C., resulting in formation of good line patterns after development. At higher temperatures, the PAGs can also undergo a thermal reaction to form a sulfonic acid. The perfluorinated substituent provides improved thermal stability and hydrolytic/nucleophilic stability.
-
Activation of sodium borohydride <i>via</i> carbonyl reduction for the synthesis of amine- and phosphine-boranes作者:P. Veeraraghavan Ramachandran、Henry J. Hamann、Randy LinDOI:10.1039/d1dt03495b日期:——carbonyl reduction by sodium borohydride is described. Unlike the prior bicarbonate-mediated protocol, which proceeds via a salt metathesis reaction, the carbon dioxide-mediated synthesis proceeds via reduction to a monoformatoborohydride intermediate. This has been verified by spectroscopic analysis, and by using aldehydes and ketones as the carbonyl source for the activation of sodium borohydride. This
表征谱图
-
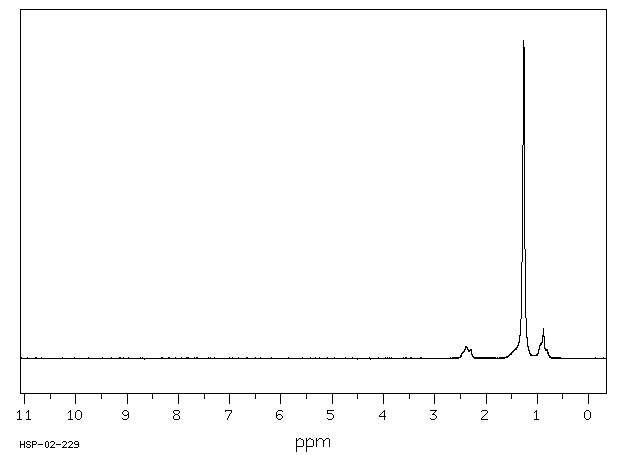
氢谱1HNMR
-
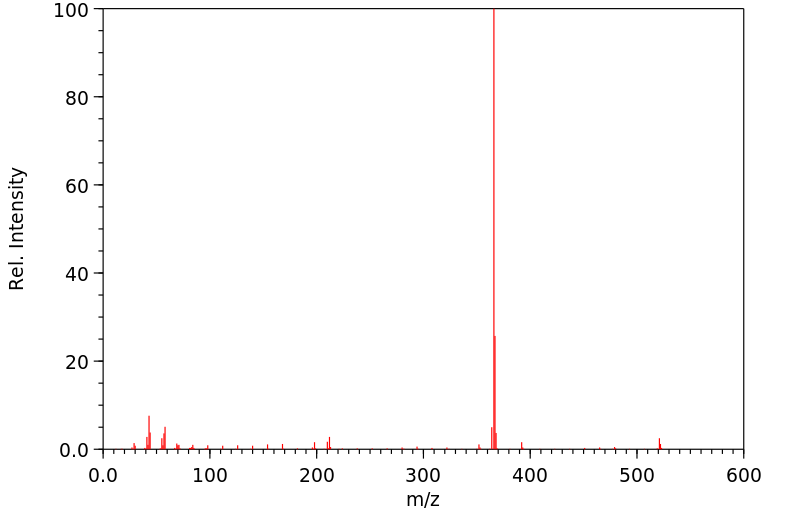
质谱MS
-
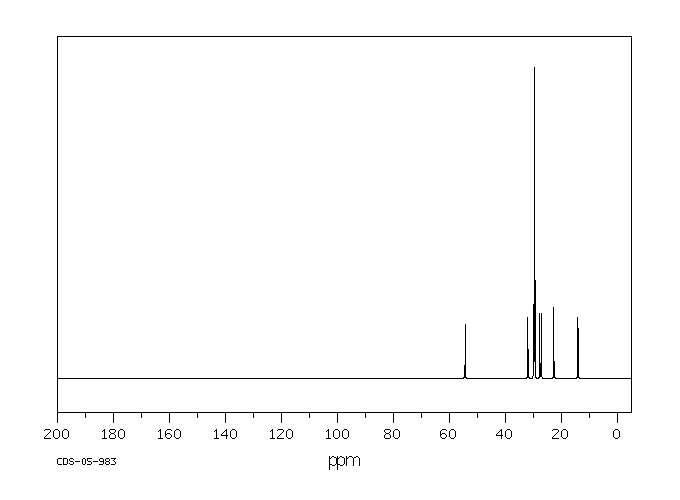
碳谱13CNMR
-
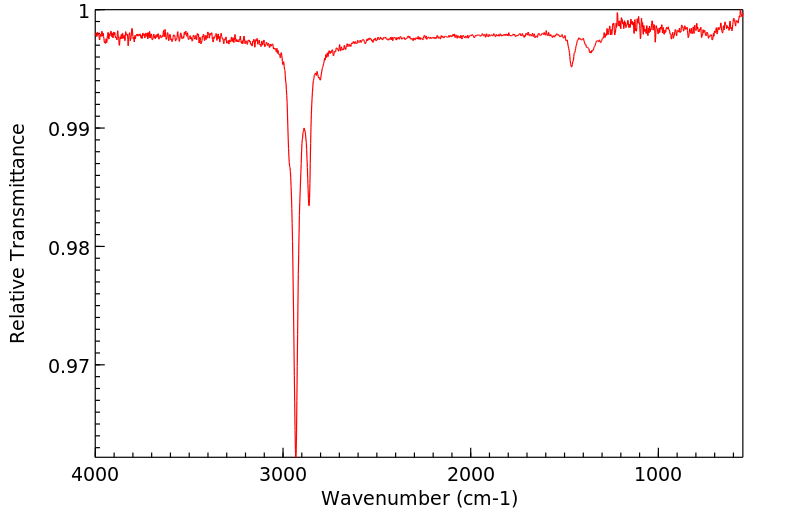
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷