1-己基-3-甲基咪唑氯化物 | 171058-17-6
中文名称
1-己基-3-甲基咪唑氯化物
中文别名
1-己基-3-甲基咪唑氯盐;1-己基-3-甲基氯化咪唑;氯化1-己基-3-甲基咪唑;1-正己基-3-甲基酰亚胺氯化物;1-己基-3-甲基咪唑鎓氯化物;1-己基-3-甲基咪唑氯
英文名称
1-hexyl-3-methylimidazol-1-ium chloride
英文别名
1-hexyl-3-methylimidazolium chloride;[hmim][Cl];[C6mim]Cl;1-n-hexyl-3-methylimidazolium chloride;1-methyl-3-hexylimidazolium chloride;3-hexyl-1-methyl-1H-imidazol-3-ium chloride;1-hexyl-3-methyl imidazole chloride;1-hexyl-3-methylimidazol-3-ium;chloride
CAS
171058-17-6
化学式
C10H19N2*Cl
mdl
——
分子量
202.727
InChiKey
NKRASMXHSQKLHA-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-85 °C
-
密度:1.0337
-
稳定性/保质期:
避氧化剂
计算性质
-
辛醇/水分配系数(LogP):-1.1
-
重原子数:13
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:8.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S23,S24/25,S26,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2933290090
-
危险性防范说明:P264,P280,P302+P352,P332+P313,P337+P313,P362+P364
-
危险性描述:H315,H319
-
储存条件:保存方法:在干燥、阴凉、密封且充满氮气的环境中存放。
SDS
1.1 产品标识符
: 1-己基-3-甲基氯化咪唑鎓
产品名称
1.2 鉴别的其他方法
HMIMCl
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: HMIMCl
别名
: C10H19ClN2
分子式
: 202.72 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
充气保存 对湿度敏感
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 1-己基-3-甲基氯化咪唑鎓
产品名称
1.2 鉴别的其他方法
HMIMCl
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: HMIMCl
别名
: C10H19ClN2
分子式
: 202.72 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氯化氢气体
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
充气保存 对湿度敏感
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
离子液体主要用于催化和无溶剂的烷基化反应,以及烯烃聚合的催化剂。
反应信息
-
作为反应物:描述:参考文献:名称:揭示温和条件下离子液体催化直接合成丙烯酸甲酯的阳离子和阴离子效应及动力学摘要:在350-380°C下由乙酸甲酯和三恶烷直接合成丙烯酸甲酯(MA)被认为是工业丙烯氧化工艺的补充途径。然而,它遭受催化剂快速失活的困扰。本文开发了一种新型的离子液体催化的温和液相体系,用于由乙酸甲酯和三恶烷直接合成MA,其中N,O-双(三甲基甲硅烷基)乙酰胺(BSA)用作α-去质子化和烯醇化的前身乙酸甲酯的甲硅烷基醚化。在[阳离子] Cl / MCl x(M = Cu +,Fe 3+,Zn 2+)的催化下,三恶烷分解为甲醛,乙酸甲酯烯化为1-甲氧基-1-三甲基甲硅烷基氧乙烯。和Al 3+)和[阳离子] F。观察到阳离子和阴离子对MA的收率和选择性有显着影响,这是由于空间位阻,酸性位点类别和吡啶探针FT-IR表征证实的强度所致。其结果是,当[N3,3,3,3] F和[N3,3,3,3] CL /产量的AlCl与MA的94.6%的选择性达60.2%是可以实现3与67摩尔%的AlCl 3分别为在DOI:10.1039/d0gc03133j
-
作为产物:描述:参考文献:名称:Various metal organic frameworks combined with imidazolium, quinolinum and benzothiazolium ionic liquids for removal of three antibiotics from water摘要:In this research, imidazolium, quinolinum and benzothiazolium based ionic liquids (ILs) were immobilized on a metal organic framework (MOF) by solvent impregnation or capillary action. The synthesized IL@MOF composite materials were characterized by FTIR, XRD, SEM and TGA methods and then applied in removal of tetracyclines (TCs) from aqueous samples. The presence of ionic liquids significantly improved the adsorption efficiency of the metal organic framework, with 82% or higher removal percentage was obtained for the three target TCs while the pristine MOB adsorption efficiency was below 50%. This could be attributed to the ability of ILs to make complex interaction with target drugs via multiple intermolecular forces. Experimental results revealed the effects of three significant factors including pH, temperature and solid-liquid ratio, and optimum adsorption efficiency could be achieved at pH 8 and 30 degrees C when solid-liquid ratio 1:2 was adopted. The adsorption kinetics was properly fitted with pseudo-second order model and Redlich-Peterson model could be used to describe the adsorption isotherm for three antibiotics; moreover, the adsorption was an endothermic and spontaneous process in nature. Finally, the adsorbed TCs could be desorbed efficiently and the performance of the IL@MOF sorbent was further verified by actual water samples. (C) 2020 Published by Elsevier B.V.DOI:10.1016/j.molliq.2020.114304
-
作为试剂:描述:2-phenyl-4-pentynoic acid 在 [C6mim][AuCl4] 、 1-己基-3-甲基咪唑氯化物 作用下, 反应 1.0h, 以96%的产率得到3-phenyl-5-methylidenetetrahydro-2-furanone参考文献:名称:Gold imidazolium-based ionic liquids, efficient catalysts for cycloisomerization of γ-acetylenic carboxylic acids摘要:离子液体稳定的氯金酸(III)被证明在无碱性条件下,对于空间位阻和非位阻的炔基羧酸底物进行环化反应时,是一种非常活跃的催化剂。DOI:10.1039/b812580e
文献信息
-
The one-pot synthesis of butyl-1H-indol-3-alkylcarboxylic acid derivatives in ionic liquid as potent dual-acting agent for management of BPH作者:Li-Yan Zeng、Fubiao Yang、Kaixuan Chen、Yunong Zeng、Zhenzhou Jiang、Shuwen Liu、Baomin XiDOI:10.1016/j.ejmech.2020.112616日期:2020.11H-indol-3-yl)butanoic acid 4aaa was designed against BPH and synthesized by two steps of N-alkylation. One-pot protocol towards 4aaa was newly developed. With IL [C6min]Br as solvent, the yield of 4aaa was increased to 75.1 % from 16.0 % and the reaction time was shortened in 1.5 hours from 48 hours. 25 Derivatives structurally based on arylpiperazine and indolyl butyric acid with alkyl linker were基于两种α的SAR 1 -AR拮抗剂和5α还原酶(5AR)抑制剂,双重作用的药剂4-(1-(4-(4-(2-甲氧基苯基)哌嗪-1-基)丁基针对BPH设计了)-1 H-吲哚-3-基)丁酸4AAA,并通过N-烷基化的两个步骤合成。新开发了针对4AAA的一锅协议。用IL [C 6分钟]溴作为溶剂,收率4AAA从16.0%提高到75.1%,反应时间是1.5小时缩短为48小时。制备了25种基于芳基哌嗪和吲哚基丁酸的具有烷基连接基的衍生物。进一步扩展了该协议,以获取另外14个衍生词,其中O-烷基化参与其中,并应用于生物有效分子DPQ和阿立哌唑的合成。预期地,化合物4AAA表现出α的双重抑制1 -AR和5α还原酶,和exihited针对人类细胞没有明显的细胞毒性。还确定了4AAA的药代动力学特性。
-
A Convenient Synthesis of Triflate Anion Ionic Liquids and Their Properties作者:Nikolai V. Ignat’ev、Peter Barthen、Andryi Kucheryna、Helge Willner、Peter SartoriDOI:10.3390/molecules17055319日期:——synthesis of high purity triflate ionic liquids via direct alkylation of organic bases (amines, phosphines or heterocyclic compounds) with methyl and ethyl trifluoromethanesulfonate (methyl and ethyl triflate) has been developed. Cheap and non-toxic dimethyl and diethyl carbonate serve as source for the methyl and ethyl groups in the preparation of methyl and ethyl triflate by this invented process. The properties
-
Multi-functional fluorinated ionic liquid infused slippery surfaces with dual-responsive wettability switching and self-repairing作者:Qingqing Rao、Ao Li、Jiawen Zhang、Jingxian Jiang、Qinghua Zhang、Xiaoli Zhan、Fengqiu ChenDOI:10.1039/c8ta08956f日期:——multiple responsive slippery surfaces based on other lubricants possessing self-repairing properties. Here, a smart slippery surface with controllable wettability was fabricated by using solid/liquid fluorinated ionic liquids (FILs) as lubricants and fluoropolymer films as the underlying substrates. The FIL, a thermo-responsive phase-transition material, was synthesized by ionic exchange and the substrate具有可控润湿性的刺激响应性光滑表面由于其在许多领域中的潜在应用而备受关注。但是,大多数这些表面仅采用石蜡作为润滑油,仅通过加热即可实现润湿性转变。此外,鲜有关于光滑滑面的自修复特性的报道。因此,在基于具有自修复特性的其他润滑剂制造多个响应性光滑表面方面仍然存在挑战。在这里,通过使用固体/液体氟化离子液体(FIL)作为润滑剂和含氟聚合物薄膜作为下层基材,制造了具有可控制的润湿性的光滑表面。FIL是一种热响应相变材料,通过离子交换法合成了碳纳米管,该基体表现出良好的自修复性能以及磁热和光热响应。通过在光照射或磁场下调节基板温度以改变FIL的相态,表面可以实现可湿性转换。此外,可以通过调节熔化温度来调节响应温度(FIL的T m)。FIL最初是合成的,没有报道使用FIL作为润滑剂来实现光滑表面的润湿性变化。这项工作为制备智能的光滑表面提供了一种新型的润滑剂,从而促进了光滑表面在液体传输设备中的应用。
-
Dialkyl Imidazolium Benzoates - Room Temperature Ionic Liquids Useful in the Peracetylation and Perbenzoylation of Simple and Sulfated Saccharides作者:Robert Linhardt、Saravanababu Murugesan、Nathalie Karst、Tasneem Islam、John WiencekDOI:10.1055/s-2003-40336日期:——Dialkyl imidazolium benzoates, room temperature ionic liquids including 1-ethyl-3-methyl imidazolium benzoate, 1-butyl-3-methyl imidazolium benzoate and 1-hexyl-3-methyl imidazolium benzoate were used in the peracetylation and perbenzoylation of several simple and sulfated carbohydrates. Organic solvents and catalysts were not required in the syntheses and these ionic liquids gave excellent yields in short reaction times.
-
Acid-induced chemoselective arylthiolations of electron-rich arenes in ionic liquids from sodium arylsulfinates: the reducibility of halide anions in [Hmim]Br
表征谱图
-
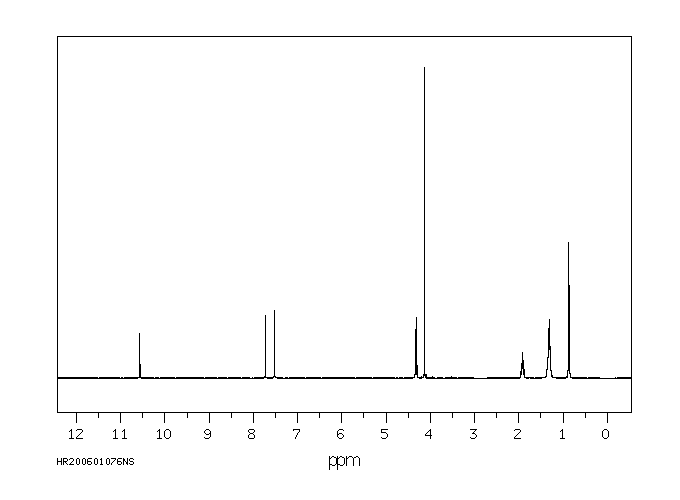
氢谱1HNMR
-
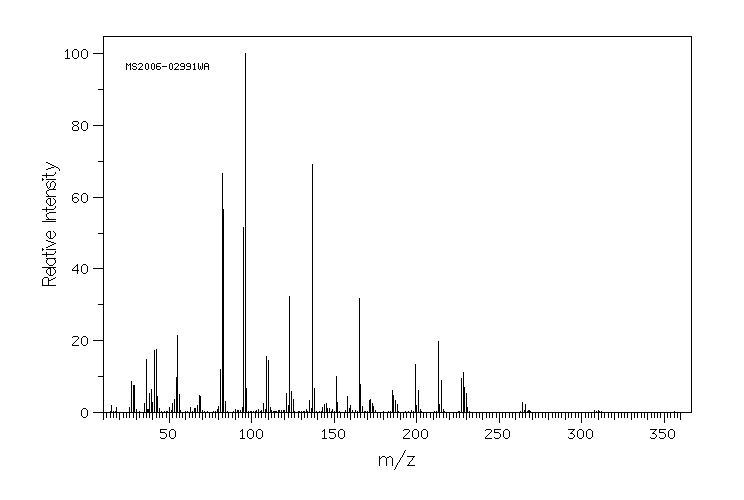
质谱MS
-
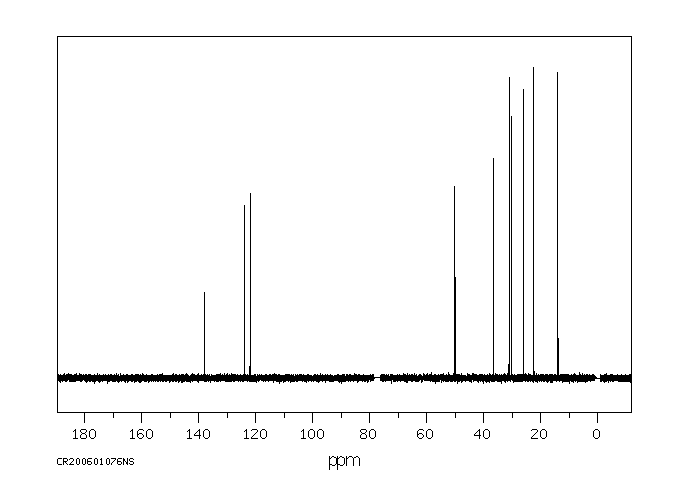
碳谱13CNMR
-
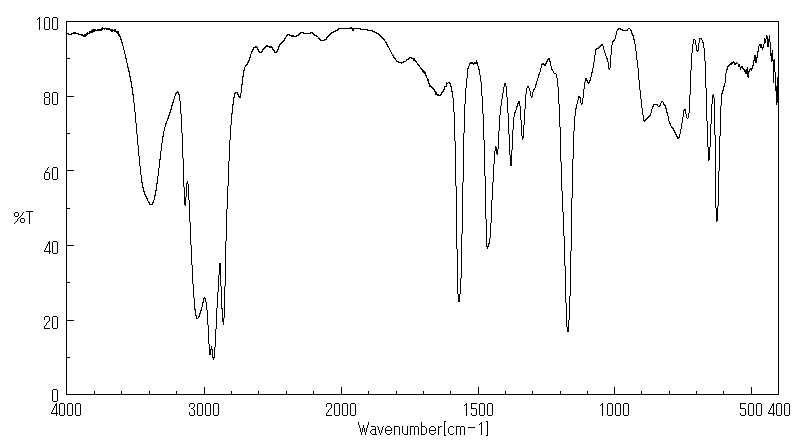
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)