1-甲基硫代-2-丙酮 | 14109-72-9
中文名称
1-甲基硫代-2-丙酮
中文别名
1-甲硫基丙酮;1-甲硫基-2-丙酮;(甲硫基)丙酮
英文名称
methylsulfanyl-acetone
英文别名
methylthioacetone;1-Methylthio-2-propanone;1-methylsulfanylpropan-2-one
CAS
14109-72-9
化学式
C4H8OS
mdl
MFCD00015325
分子量
104.173
InChiKey
UKFADLGENFFWHR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:69-70°C 30mm
-
密度:1,023 g/cm3
-
闪点:52°C
-
溶解度:氯仿(微溶)、甲醇(微溶)
-
LogP:0.49
-
物理描述:colourless to pale yellow liquid with odour of melon
-
折光率:1.395-1.405
-
保留指数:863;863
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:42.4
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:3
-
安全说明:S23,S24/25
-
危险类别码:R10
-
危险品运输编号:1224
-
海关编码:2930909090
-
包装等级:III
-
危险类别:3
-
储存条件:常温下应密闭避光保存,并保持通风和干燥。
SDS
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Boehme,H.; Krack,W., Justus Liebigs Annalen der Chemie, 1977, p. 51 - 60摘要:DOI:
-
作为产物:描述:参考文献:名称:Kirrmann,A. et al., Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences, 1962, vol. 255, p. 728 - 730摘要:DOI:
文献信息
-
Asymmetric Organocatalytic Direct Aldol Reactions of Ketones with α-Keto Acids and Their Application to the Synthesis of 2-Hydroxy-γ-butyrolactones作者:Xiao-Ying Xu、Zhuo Tang、Yan-Zhao Wang、Shi-Wei Luo、Lin-Feng Cun、Liu-Zhu GongDOI:10.1021/jo701868t日期:2007.12.1of organocatalysts for the asymmetric direct aldol reactions of ketones with α-keto acids were designed on the basis of molecular recognition and prepared from proline and aminopyridines. The organic molecule 8e, derived from proline and 6-methyl-2-amino pyridine, was the best catalyst, affording excellent enantioselectivities (up to 98% ee) for the direct aldol reactions of acetone or 2-butanone with
-
Asymmetric Reduction of 1-Acetoxy-2-alkanones with Bakers’ Yeast: Purification and Characterization of<i>α</i>-Acetoxy Ketone Reductase作者:Kohji Ishihara、Nobuyoshi Nakajima、Sadao Tsuboi、Masanori UtakaDOI:10.1246/bcsj.67.3314日期:1994.12An α-acetoxy ketone reducing enzyme has been purified and characterized from the cell-free extract of bakers’ yeast (Saccharomyces cerevisiae). Only one NADPH-dependent dehydrogenase that catalyzed the reduction of α-acetoxy ketone was found in bakers’ yeast. The molecular weight of the enzyme was estimated to be 36 kDa by SDS-polyacrylamide gel electrophoresis. The enzyme was composed of a single
-
Aldol additions of pinacolone lithium enolate with ketones: reactivities governed predominantly by field effects作者:Goutam Das、Edward R. ThorntonDOI:10.1021/ja00057a012日期:1993.2The relative reactivities of representative α- and β-heterosubstituted acyclic, cyclic (five- and six-membered), and aromatic ketones with the lithium enolate of pinacolone in diethyl ether at -78 o C were determined. The order of reactivitiesof monosubstituted acetones (MeCOCH 2 X) is X=Cl>OTBDMS>OMe>SMe>NMe 2 >CH 2 SM>H>Me and spans a range of 10 4
-
Synthesis of indoles from anilines and intermediates therein申请人:The Ohio State University Research Foundation公开号:US03992392A1公开(公告)日:1976-11-16Preparing indoles and intermediates therefor by reacting an N-haloaniline with a .beta.-carbonylic hydrocarbon sulfide to form an azasulfonium halide, reacting the azasulfonium halide with a strong base to form a thio-ether indole derivative, and then reducing the thio-ether indole, e.g. with Raney nickel, to form the indole compound. When an acetal or ketal of the .beta.-carbonyl hydrocarbon sulfide is used, the azasulfonium salt is treated with a base, and then with an acid to form the thio-ether indole derivative. When an .alpha.-ethyl-.beta. -carbonylic hydrocarbon sulfide is used, the resulting azosulfonium salt reacts with strong base to form a thio-ether indolenine derivative, which on reduction with Raney nickel or complex metal hydrides yields 3-substituted indoles. The aniline may be an aminopyridine to form an aza-indole compound in the process. The azasulfonium salts and thio-ether indole or thio-ether indolenine derivatives can be isolated and recovered from their respective reaction mixtures. The thio-ether-indole and thio-ether indolenine derivatives are useful as intermediates to make the indoles without the thio-ether group. The indoles are known compounds having a wide variety of uses, e.g., in making perfumes, dyes, amino acids, pharmaceuticals, agricultural chemicals and the like.通过将N-卤苯胺与β-羰基碳氢砜反应以形成氮硫杂环卤化物,然后将氮硫杂环卤化物与强碱反应以形成硫醚吲哚衍生物,然后还原硫醚吲哚,例如使用雷内镍,以形成吲哚化合物。当使用β-羰基碳氢砜的缩醛或缩酮时,处理氮硫杂环盐与碱,然后与酸反应以形成硫醚吲哚衍生物。当使用α-乙基-β-羰基碳氢砜时,生成的氮硫杂环盐与强碱反应以形成硫醚吲哚啉衍生物,然后通过雷内镍或复杂金属氢化物还原,得到3-取代吲哚。苯胺可以是氨基吡啶,以在过程中形成氮杂吲哚化合物。氮硫杂环盐和硫醚吲哚或硫醚吲哚啉衍生物可以从各自的反应混合物中分离和回收。硫醚吲哚和硫醚吲哚啉衍生物可用作制备不含硫醚基团的吲哚的中间体。吲哚是已知化合物,具有各种用途,例如制造香水、染料、氨基酸、药品、农药等。
-
Mono- and polynuclear complexes from the reaction of palladium acetate with α-substituted thioethers and thiols作者:Marino Basato、Diego Tommasi、Marco ZeccaDOI:10.1016/s0022-328x(98)00910-3日期:1998.11trimers decreases with the steric hindrance of the substituents at the sulphur and at the methine carbon atoms. Stable mixed sphere complexes are obtained also with carboxylato ligands different from acetato as PhCOO− and MeSCH2COO−. When the substituted thioether has poor electronwithdrawing groups, its reaction with palladium acetate affords complexes of the type [Pd(η1-OAc)2(RSCH2Z)2], in which the sulphur乙酸钯与α-取代的硫醚(RSCH(R')Z; Z =酯,酮,砜,取代的甲基)和硫醇HSCH 2 C(O)Me反应生成化合物,其组成和核数主要取决于电子性质硫配体。如果它包含足够酸性的氢原子,则乙酰基部分或全部作为乙酸被除去,从而生成[Pd 3(μ- O 2 CMe)3(μ- RSCR'Z)3 ]和[Pd(SCH)2 C(O)Me)2 ] 6。三聚体的稳定性随着取代基在硫和次甲基碳原子上的空间位阻而降低。稳定的混合球复合物与羧酸根也获得配体从如乙酸根不同PhCOO -和MeSCH 2 COO - 。当取代的硫醚具有差的吸电子基团,其与该类型的乙酸钯,得到配合物[钯(反应η 1 -OAc)2(RSCH 2 Z)2 ],其中,所述硫给体原子已经简单地更换的一个氧原子乙酰基配体。
表征谱图
-
氢谱1HNMR
-
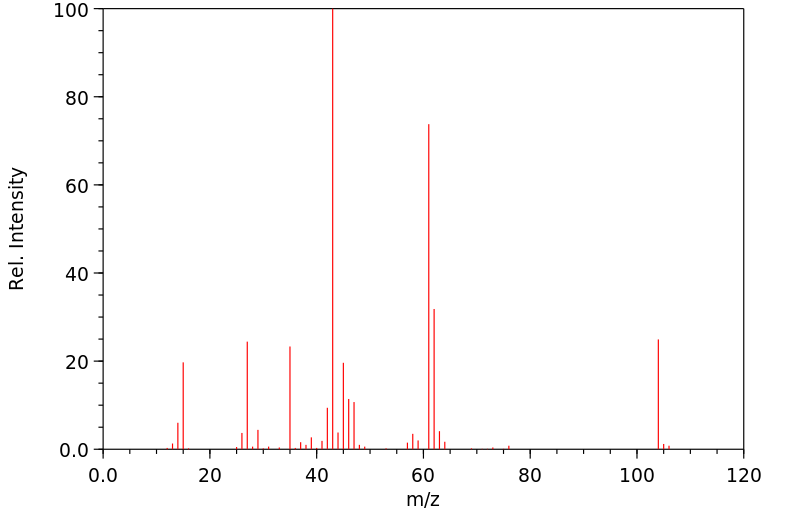
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷