二氯化乙基磷酸 | 1066-50-8
中文名称
二氯化乙基磷酸
中文别名
——
英文名称
Ethylphosphonic dichloride
英文别名
1-dichlorophosphorylethane
CAS
1066-50-8
化学式
C2H5Cl2OP
mdl
——
分子量
146.941
InChiKey
OWGJXSYVHQEVHS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:71-72 °C12 mm Hg(lit.)
-
密度:1.376 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
溶解度:易溶于氯仿和二氯甲烷。
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:8
-
危险品标志:T
-
安全说明:S23,S26,S36/37/39,S45
-
危险类别码:R23/24/25,R34
-
WGK Germany:3
-
RTECS号:TA1780000
-
海关编码:2931900090
-
包装等级:II
-
危险类别:8
-
危险品运输编号:UN 2922 8/PG 2
-
储存条件:请将容器密封保存,并存放在阴凉干燥处。
SDS
| Name: | Ethylphosphonic dichloride 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1066-50-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1066-50-8 | Ethylphosphonic dichloride | 98% | 213-918-3 |
Risk Phrases: 20/21/22 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed. Causes burns.Moisture sensitive.Corrosive.
Potential Health Effects
Eye:
Causes eye burns.
Skin:
Harmful if absorbed through the skin. Causes skin burns.
Ingestion:
Harmful if swallowed. May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Harmful if inhaled. May cause irritation of the respiratory tract with burning pain in the nose and throat, coughing, wheezing, shortness of breath and pulmonary edema. Causes chemical burns to the respiratory tract. Inhalation may be fatal as a result of spasm, inflammation, edema of the larynx and bronchi, chemical pneumonitis and pulmonary edema.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed.
Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Wash mouth out with water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Reacts violently with water giving off flammable gas which may explode. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide. DO NOT USE WATER! Do NOT get water inside containers.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation. Do not get water inside containers.
Section 7 - HANDLING and STORAGE
Handling:
Do not breathe dust, vapor, mist, or gas. Keep container tightly closed. Do not get on skin or in eyes. Do not ingest or inhale. Use with adequate ventilation. Do not allow contact with water. Use only in a chemical fume hood. Discard contaminated shoes. Keep from contact with moist air and steam.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Store in a tightly closed container. Keep under a nitrogen blanket.
Corrosives area. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1066-50-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin. Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 71 - 72 deg C @ 12.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.3760g/cm3
Molecular Formula: C2H5P(O)Cl2
Molecular Weight: 146.94
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials, moisture.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Hydrogen chloride, phosphine, carbon monoxide, oxides of phosphorus, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1066-50-8: TA1780000 LD50/LC50:
Not available.
Carcinogenicity:
Ethylphosphonic dichloride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S.*
Hazard Class: 8
UN Number: 3265
Packing Group: II
IMO
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3265
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE LIQUID, ACIDIC, ORGANIC, N.O.S.
Hazard Class: 8
UN Number: 3265
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
R 34 Causes burns.
Safety Phrases:
S 25 Avoid contact with eyes.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1066-50-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1066-50-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1066-50-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:在气相色谱/质谱分析条件下,CWC相关异构的二烷基烷基硫代磷酸二烷基酯/硫代磷酸烷基酯的质谱表征。摘要:理由禁化武组织实施的《化学武器公约》(CWC)的列入清单的化学品列为异构的二烷基烷基硫代磷酸二烷基酯和烷基烷基膦酸二烷基酯。必须正确标识PS和PR键的连接性,以验证CWC。本研究证明了在电子电离(EI)或化学电离(CI)条件下通过选择性裂解成功鉴定目标异构体。方法在我们的实验室中,使用既定方法合成了所有研究的异构化合物(共27种),然后使用配备HP-5MS毛细管柱的Agilent 6890气相色谱仪通过EI和CI气相色谱/质谱(GC / MS)进行分析并连接到5973 N质量选择检测器。使用范登杜尔公式计算所有化合物的保留指数(RI)值。还分别使用VG-Autospec(磁性扇区)和JEOL-AccuToF(飞行时间)质谱仪进行了GC / MS / MS和GC / HRMS实验。结果所有化合物的EI质谱在m / z 182处均具有丰富的分子离子,除了少数几种丁基取代的化合物(该离子的丰度较DOI:10.1002/rcm.6596
-
作为产物:参考文献:名称:合成有用的膦酸二氯化物的温和制备:在合成环状膦酸二酯和二酰胺中的应用摘要:已经开发了一种非常温和的制备合成上有用的二氯化膦的方法,并将其用于合成2-烷基-2-氧代-1、3、2-二氧代膦酰基和1,3-二甲基-2-烷基-2-基。报导了oxo-1、3、2-二氮杂膦烷。DOI:10.1016/s0040-4039(00)89038-3
-
作为试剂:描述:参考文献:名称:Martynov,I.V. et al., Journal of general chemistry of the USSR, 1967, vol. 37, # 5, p. 1072 - 1073摘要:DOI:
文献信息
-
Process for the preparation of phosphonic acid dihalides申请人:Hoechst Aktiengesellschaft公开号:US03972923A1公开(公告)日:1976-08-03Process for preparing phosphonic acid dihalides of the formula ##EQU1## wherein R is alkyl of 1 to 18 carbon atoms, cycloalkyl of 4 to 8 carbon atoms, alkenyl of 2 to 18 carbon atoms, phenyl, phenalkyl or alkyl-phenyl of 7 to 8 carbon atoms, phenyl, phenalkyl or alkyl-phenyl of 7 to 8 carbon atoms, all radicals R optionally being substituted by chlorine, bromine, cyano or lower acyloxy, and wherein X is halogen such as 2-chloroethane phosphonic acid dichloride, by reacting phosphonic or thio-phosphonic acids of the formula ##EQU2## wherein Y is oxygen or sulfur, their salts or functional derivatives, with acid halides of the formula (CO).sub.n X.sub.2 (III) wherein X is chlorine or bromine and n is 1 or 2, in the presence of 0.2 - 5% or in the presence of 0.01 to 0.2% by weight of 1. compounds containing at least one tri- to pentavalent nitrogen or phosphorus atom, which in the case of nitrogen is bound with 1 to 4, in the case of phosphorus with at least 3 valences to organic radicals having up to 20 carbon atoms, two of these valences optionally forming a double bond, or 2. mono-di- or tribasic organic or inorganic fully amidated acids or tri- or pentavalent phosphorus, the N atom of which optionally being substituted by aliphatic radicals having up to 20 carbon atoms, and the organic radicals of which contain up to 20 carbon atoms, If required, in the presence of an inert solvent.制备式为##EQU1##的膦酸二卤代物的过程,其中R是1至18个碳原子的烷基,4至8个碳原子的环烷基,2至18个碳原子的烯基,苯基,苯基烷基或7至8个碳原子的烷基苯基,苯基,苯基烷基或7至8个碳原子的烷基苯基,所有基团R可选择地被氯,溴,氰基或较低的酰氧基取代,其中X是卤素,例如2-氯乙烷膦酸二氯化物,通过将式为##EQU2##的膦酸或硫膦酸与式为(CO).sub.nX.sub.2(III)的酸卤反应而得,其中Y是氧或硫,它们的盐或功能衍生物,X是氯或溴,n为1或2,在0.2-5%的存在下或在0.01至0.2%的存在下按重量计的1.至少含有一个三至五价氮或磷原子的化合物,其中在氮的情况下与1至4个,在磷的情况下与至少3个价的有机基团结合,这些价中的两个可选择地形成双键,或2.单,二或三碱基的有机或无机全酰胺酸或三价或五价磷,其中N原子可选择地被含有最多20个碳原子的脂肪基团取代,其有机基团含有最多20个碳原子,如有必要,在惰性溶剂的存在下。
-
The Mechanism of Thionyl Chloride Reaction with Dialkyl Alkylphosphonothionate Using 31 P NMR作者:Purnanand、P. D. Shakya、Shefali Saxena、M. Sharma、Basant LalDOI:10.1080/10426500211715日期:2002.5.1Based on 31 P NMR studies of thionyl chloride reaction with dialkyl alkylphosphonothionates, a method for preparation of alkylphosphonic dichloride has been investigated. A mechanism via intermediacy of ester chloride is suggested.
-
Hydrolysis of cyclic phosphoramides. Evidence for syn lone pair catalysis作者:Andrés Núñez、Dyanna Berroterán、Oswaldo NúñezDOI:10.1039/b300916e日期:——Hydrolysis between 1.5 < pH < 4 of five and six membered cyclic phosphoramides has been followed by UV and 31PNMR spectroscopy. The observed rates fit the equation: kobs = kH2O [H+]/([H+] + Ka) + k′H2O, where kH2O and k′H2O are the pseudo first-order rate constants of water attack on the protonated phosphoramide and its unprotonated form, respectively, and Ka is the phosphoramide acidity equilibrium constant. Although, faster hydrolysis rates on the five membered ring are expected due to the energy released in going from a strained cyclic to a “strained free” trigonal-bipyramidal-pentacoordinated intermediate, with one of the cyclic nitrogens occupying the apical position, these compounds react slightly faster (kH2O values) but slower regarding the k′H2O values than the six membered analogs. The balance in reactivity is attributed to the additional stability obtained in the six membered cyclic compounds by a syn orientation of the two lone pairs of the cyclic nitrogen to the water attack. This stabilization does not exist in the five membered phospholidines since the water attack is perpendicular to the electron pairs of the cyclic nitrogen. In agreement with the incoming water orientation, the product ratios from the hydrolysis show that in the five membered rings the main product is the one produced by endocyclic cleavage; meanwhile, in the six membered cyclic phospholines the kinetic product is the one produced by exocyclic cleavage. The syn orientation of two electron pairs on nitrogen stabilizes the transition state of water approach to the phosphoramides by ca. 3 kcal mol−1 when compared to the orthogonal attack.在1.5 < pH < 4的条件下,五元环和六元环磷酰胺的水解过程通过紫外光谱和31P核磁共振波谱进行了研究。观察到的反应速率符合以下方程:kobs = k [H+]/([H+] + Ka) + k′H2O,其中k 和k′ 分别是水分子攻击质子化磷酰胺和非质子化磷酰胺的假一级速率常数,Ka是磷酰胺酸度平衡常数。虽然预计五元环的水解速率会更快,因为从受限环状结构转变为“无受限”三角双锥五配位中间态时释放能量,其中一个环状氮原子占据顶端位置,但这些化合物的反应速率(k 值)略快,而关于k′ 值则较慢于六元环的类似物。反应活性的平衡归因于六元环状化合物在水攻击时通过syn取向使环状氮的两个孤对电子与水攻击方向一致所获得的额外稳定性。这种稳定化在五元环磷酯中不存在,因为水攻击方向垂直于环状氮的电子对。与进来的水取向一致,水解产物的比例表明,在五元环中主要产物是由环内断裂产生的;而在六元环磷脂中,动力学产物是由环外断裂产生的。氮原子上两个电子对的syn取向通过与正交攻击相比,大约稳定了磷酰胺水接近过渡态的3 kcal mol−1。
-
THE CONDENSATION OF PHOSPHONOTHIOIC AND PHOSPHONIC DICHLORIDES WITH <i>o</i>-DIAMINES作者:Ralph L. Dannley、Arturs GravaDOI:10.1139/v65-469日期:1965.12.1dichlorides, and phenylenediamines containing electron-donating substituents are more reactive than those containing electron-withdrawing substituents. The diazaphosphole 2-oxides undergo hydrolysis or alcoholysis of only one of the amide groups under mild conditions. The 2-sulfides are much more resistant to hydrolysis than the 2-oxides. The 2-sulfides are converted to the N-methyl derivatives by dimethyl
-
Stereoselective inhibition of glutamate carboxypeptidase by organophosphorus derivatives of glutamic acid作者:Jeremy P. Mallari、Cindy J. Choy、Ying Hu、Alicia R. Martinez、Mia Hosaka、Yoko Toriyabe、Jack Maung、Joseph E. Blecha、Stephen F. Pavkovic、Clifford E. BerkmanDOI:10.1016/j.bmc.2004.08.016日期:2004.11stereoselective inhibition consistent with the configuration of the chiral phosphorus center, this effect was generally not remarkable. More important, was the effect of carbon stereochemistry upon glutamate carboxypeptidase inhibition as exemplified by a limited series of enantiomeric pairs of phosphonamidate and phosphonamidodithionate derivatives of glutamic acid. The phosphonamidate analogs derived制备一系列(S)-和(R)-谷氨酸的烷基和芳基膦酰基,硫代膦酰基和二硫代膦酰基衍生物,并检查其对谷氨酸羧肽酶(羧肽酶G)的抑制能力。磷酸亚氨基二硫代酸和单个磷酸氨基硫代酸非对映异构体的获得是通过常见的氨基磷酸硫代氨基甲酸酯前体实现的,该前体也允许对磷酸氨基硫代酸的手性磷中心进行色谱拆分。该系列中最有效的抑制剂是谷氨酸天然异构体的正丁基膦酰胺酸酯衍生物。尽管每对非对映异构体对三个磷酸氨基硫代酸酯都显示出与手性磷中心的构型一致的立体选择性抑制,但这种效果通常并不明显。更重要的是,碳立体化学对谷氨酸羧肽酶抑制的影响,如谷氨酸的膦酰胺和膦酰胺二硫代酸酯衍生物的一系列对映异构体对所例示的。与它们的对映异构体相反,衍生自谷氨酸的非天然立体异构体的膦酰胺类似物没有抑制效力。令人惊讶地,衍生自谷氨酸的非天然立体异构体的磷酸氨基二硫代磷酸酯显示出比其天然来源的对映体更大的抑制效力。与它们的对映异构体相反,衍
表征谱图
-
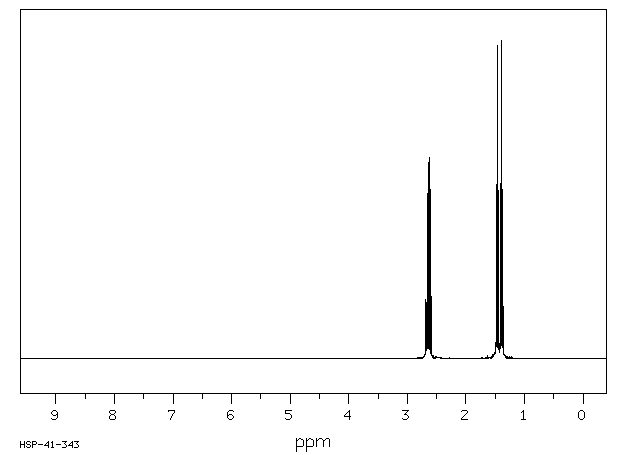
氢谱1HNMR
-
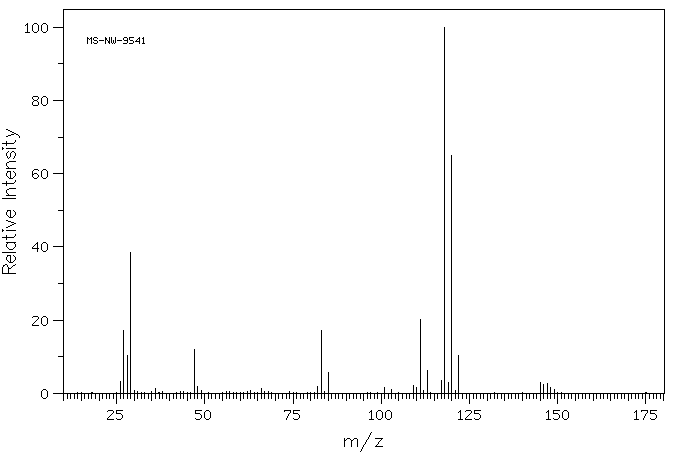
质谱MS
-
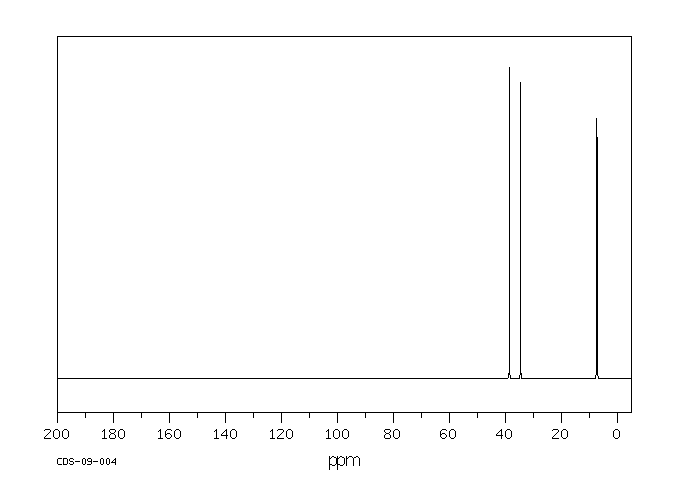
碳谱13CNMR
-
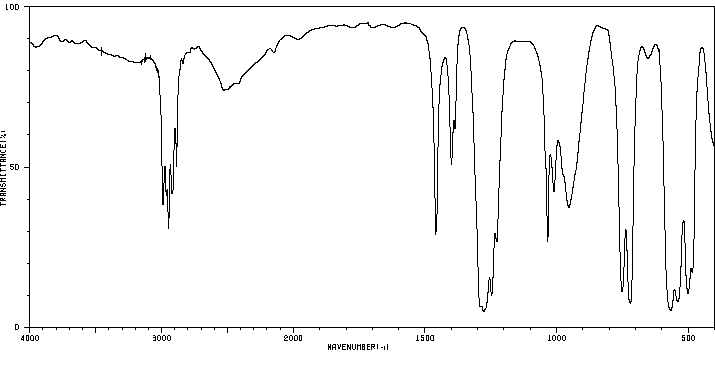
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1-氨基丁基)磷酸
顺丙烯基磷酸
除草剂BUMINAFOS
阿仑膦酸
阻燃剂 FRC-1
铵甲基膦酸盐
钠甲基乙酰基膦酸酯
钆1,5,9-三氮杂环十二烷-N,N',N''-三(亚甲基膦酸)
钆-1,4,7-三氮杂环壬烷-N,N',N''-三(亚甲基膦酸)
重氮甲基膦酸二乙酯
辛基膦酸二丁酯
辛基膦酸
辛基-膦酸二钾盐
辛-1-烯-2-基膦酸
试剂12-Azidododecylphosphonicacid
英卡膦酸
苯胺,4-乙烯基-2-(1-甲基乙基)-
苯甲基膦酸二甲酯
苯基膦酸二甲酯
苯基膦酸二仲丁酯
苯基膦酸二乙酯
苯基膦酸二乙酯
苯基磷酸二辛酯
苯基二异辛基亚磷酸酯
苯基(1H-1,2,4-三唑-1-基)甲基膦酸二乙酯
Tetrapotassium (((2-hydroxyethyl)imino)bis(methylene))bisphosphonate
苄基膦酸苄基乙酯
苄基亚甲基二膦酸
膦酸,[(2-乙基己基)亚氨基二(亚甲基)]二,triammonium盐(9CI)
膦酸叔丁酯乙酯
膦酸单十八烷基酯钾盐
膦酸二辛酯
膦酸二(二十一烷基)酯
膦酸,辛基-,单乙基酯
膦酸,甲基-,单(2-乙基己基)酯
膦酸,甲基-,二(苯基甲基)酯
膦酸,甲基-,2-甲氧基乙基1-甲基乙基酯
膦酸,丁基乙基酯
膦酸,[苯基[(苯基甲基)氨基]甲基]-,二甲基酯
膦酸,[[羟基(苯基甲基)氨基]苯基甲基]-,二(苯基甲基)酯
膦酸,[2-(环丙基氨基)-2-羰基乙基]-,二乙基酯
膦酸,[2-(二甲基亚肼基)丙基]-,二乙基酯,(E)-
膦酸,[1-甲基-2-(苯亚氨基)乙烯基]-,二乙基酯
膦酸,[1-(乙酰基氨基)-1-甲基乙基]-(9CI)
膦酸,[(环己基氨基)苯基甲基]-,二乙基酯
膦酸,[(二乙氧基硫膦基)(二甲氨基)甲基]-
膦酸,[(2S)-2-氨基-2-苯基乙基]-,二乙基酯
膦酸,[(1Z)-2-氨基-2-(2-噻嗯基)乙烯基]-,二乙基酯
膦酸,P-[(二乙胺基)羰基]-,二乙基酯
膦酸,(氨基二环丙基甲基)-