(S)-2-氨基-2-甲基丁酸 | 595-39-1
中文名称
(S)-2-氨基-2-甲基丁酸
中文别名
DL-2-氨基-2-甲基丁酸
英文名称
DL-isovaline
英文别名
Isovalin;isovaline;DL-Isovalin;DL-α-Amino-α-methyl-buttersaeure;2-Amino-2-methyl-buttersaeure;L-2-methyl-2-aminobutanoic acid;2-azaniumyl-2-methyl-butanoate;D-Isovaline;2-azaniumyl-2-methylbutanoate
CAS
595-39-1
化学式
C5H11NO2
mdl
MFCD00272968
分子量
117.148
InChiKey
GCHPUFAZSONQIV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:307-308° (closed tube)
-
沸点:214℃
-
密度:1.070
-
闪点:83℃
计算性质
-
辛醇/水分配系数(LogP):-2.3
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:63.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2922499990
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:应存放在室温环境下,避免光照,并在惰性气体氛围中保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Amino-2-methylbutyric acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Amino-2-methylbutyric acid
CAS number: 595-39-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C5H11NO2
Molecular weight: 117.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Amino-2-methylbutyric acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Amino-2-methylbutyric acid
CAS number: 595-39-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C5H11NO2
Molecular weight: 117.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异缬氨酸乙酯 ethyl 2-amino-2-methylbutanoate 56247-82-6 C7H15NO2 145.202 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 D(-)-异缬氨酸 (2R)-2-amino-2-methylbutanoic acid 3059-97-0 C5H11NO2 117.148 L-异缬氨酸 L-isovaline 595-40-4 C5H11NO2 117.148 2-氨基-2-甲基丁酸甲酯 methyl 2-amino-2-methylbutanoate 87162-70-7 C6H13NO2 131.175 异缬氨酸乙酯 ethyl 2-amino-2-methylbutanoate 56247-82-6 C7H15NO2 145.202 N-甲酰基-L-异缬氨酸 (+)-N-Formyl-isovalin 29588-12-3 C6H11NO3 145.158 —— (+/-)-2-formylamino-2-methyl-butyric acid 29588-10-1 C6H11NO3 145.158 1-丁醇,2-氨基-2-甲基- (R)-2-amino-2-methylbutan-1-ol 10196-30-2 C5H13NO 103.164
反应信息
-
作为反应物:描述:参考文献:名称:Auterhoff; Hansen, Pharmazie, 1970, vol. 25, # 5, p. 336 - 340摘要:DOI:
-
作为产物:描述:benzyl 2-hydroxy-2-methylbutanoate 在 palladium 10% on activated carbon 、 二(对硝基苯基)叠氮膦酸酯 、 氢气 、 1,8-二氮杂双环[5.4.0]十一碳-7-烯 作用下, 以 甲醇 、 甲苯 为溶剂, 反应 19.0h, 生成 (S)-2-氨基-2-甲基丁酸参考文献:名称:季碳中心的S N 2置换:α,α-二取代的α-氨基酸合成的新途径摘要:已开发出一种使用双(对硝基苯基)磷叠氮酸酯在季碳原子上进行S N 2反应的新方法。手性叔醇被完全转化为构型,直接转化为相应的手性叔叠氮化物。通过催化氢化将叠氮化物转化为相应的胺,可以制备几种α,α-二取代的α-氨基酯或氨基酸。DOI:10.1016/j.tetlet.2015.01.041
文献信息
-
Highly Diastereoselective Synthesis of 4-N-Methylformamidino Trinem (GV129606), a Potent Antibacterial Agent作者:Stefano Biondi、Angelo Pecunioso、Filippo Busi、Stefania A Contini、Daniele Donati、Micaela Maffeis、Domenica A Pizzi、Luciana Rossi、Tino Rossi、Fabio M SabbatiniDOI:10.1016/s0040-4020(00)00414-2日期:2000.7In this paper a highly diastereoselective synthesis of 4-N-methylformamidino trinem 3 is reported. The route offers advantages compared to that previously used, i.e. the higher overall yield, the robustness, the avoidance of toxic reagents. Most of the compounds were isolated by precipitation, therefore reducing the number of chromatographic separations. The efficient conversion of 4-N-methylamino
-
Reversed-phase liquid chromatographic resolution of diastereomers of protein and non-protein amino acids prepared with newly synthesized chiral derivatizing reagents based on cyanuric chloride作者:Ravi Bhushan、Charu AgarwalDOI:10.1007/s00726-010-0650-z日期:2011.2replaced with piperidine, and the second Cl atom in the 6-piperidinyl derivative was replaced with amino acid amide (viz l-Leu–NH2) and amino acid (l-Leu). These reagents were characterized and used as CDRs for chiral separation of protein and non-protein amino acids, and were separated on a reversed-phase C18 column. The reaction conditions were optimized for the synthesis of diastereomers using one两个新的手性单氯小号-triazines(MCT)的合成[即Ñ - (4-氯-6- piperidinyl- [1,3,5] -三嗪-2-基) -升-亮氨酰胺和ñ - (4- -氯-6-哌啶基-[1,3,5]-三嗪-2-基)-1-亮氨酸(分别为CDR 1和2)]是通过s-三嗪部分中氯原子的亲核置换实现的。一个Cl原子被哌啶取代,6-哌啶基衍生物中的第二个Cl原子被氨基酸酰胺(vil 1 - Leu -NH 2)和氨基酸(l-Leu)。对这些试剂进行了表征,并用作用于手性分离蛋白质和非蛋白质氨基酸的CDR,并在反相C 18色谱柱上进行了分离。使用一种MCT试剂优化了反应条件以合成非对映异构体。验证了分离方法的检出限,线性,准确性,精密度和回收率。
-
Does Type of Disease Matter? Gender Differences Among Alzheimer's and Parkinson's Disease Spouse Caregivers作者:Karen Hooker、Margaret Manoogian-O'Dell、Deborah J. Monahan、Leslie D. Frazier、Kim ShifrenDOI:10.1093/geront/40.5.568日期:2000.10.1Purpose of study: Mental health outcomes are widely reported among spouse caregivers, with wives generally faring worse than husbands. We hypothesized that gender differences would not be as strong in a cognitively intact group because caring for cognitively intact spouses may involve less severe reciprocity losses. We also examined gender differences in coping strategies within each group. Design and method: 175 spouse caregivers for patients with Alzheimer's disease (AD; n = 88) and Parkinson's disease (PD; n = 87) were interviewed. Participants completed perceived stress (PSS), depression (CES-D), state anxiety (STAI, Form Y), and coping strategies (WCCL-R) measures. Results:Wives in the AD group reported significantly worse mental health outcomes than husbands, while wives and husbands in the PD group showed no differences. AD caregiving wives were less likely than husbands to use problem-focused coping strategies. There were no significant gender differences in either group for social support or emotion-focused coping. Implications:Loss of reciprocity in marital relationships may affect women more negatively than men. Future studies that address underlying mechanisms of gender differences and focus on similar caregiving situations and contexts deserve attention.研究目的:配偶照护者在心理健康方面面临的问题已被广泛报道,但往往妻子比丈夫受到的影响更大。我们假设在认知功能完好的照护者中,性别差异不会那么强烈,因为照护认知完好的配偶可能涉及较少的互惠性损失。我们还探讨了每个组别中性别在应对策略上的差异。设计和方法:对175名阿尔茨海默病(AD,n=88)和帕金森病(PD,n=87)患者的配偶照护者进行了访谈。参与者完成了感知压力(PSS)、抑郁(CES-D)、状态焦虑(STAI,Y型)和应对策略(WCCL-R)的测量。结果:AD组的妻子报告了比丈夫更差的心理健康结果,而PD组的妻子和丈夫之间没有差异。AD照护妻子比丈夫更不可能使用问题焦点应对策略。在社交支持或情绪焦点应对方面,两个组别均未发现显著的性别差异。启示:婚姻关系中的互惠性损失可能对女性的负面影响大于男性。未来研究应关注性别差异的潜在机制,并聚焦于类似照护情境和背景的探讨。
-
Derivatization and Multidimensional Gas-Chromatographic Resolution of α-Alkyl and α-Dialkyl Amino Acid Enantiomers作者:Cornelia Meinert、Uwe J. MeierhenrichDOI:10.1002/cplu.201300328日期:2014.6amino acids in two chromatographic dimensions is reported. It was found that enantiomers of α‐dialkyl amino acids can be readily resolved with the use of an enantioselective Chirasil‐Val stationary phase in the first chromatographic dimension and a polyethylene glycol stationary phase in the second dimension. These data offer new possibilities for the enantioselective resolution and precise quantification氨基酸对映体的定量拆分对于许多生物分析化学领域至关重要,包括食品和植物分析,代谢组学,药物化学和考古化学。然而,已证明对α-二烷基氨基酸的对映选择性分析要比对蛋白质质子化的α-质子化氨基酸困难。本文报道了一种能够在二维色谱中对α-烷基和α-二烷基氨基酸对映体进行基线分离的分析方法的开发。已经发现,通过在第一色谱方面使用对映选择性Chirasil-Val固定相并在第二维使用聚乙二醇固定相,可以轻松拆分α-二烷基氨基酸的对映异构体。这些数据为非生物α-二烷基氨基酸的对映选择性拆分和精确定量化提供了新的可能性。随后,该方法将特别应用于对与了解地球上同手性起源有关的样品(如陨石,彗星和星际冰)的对映选择性分析。
-
Stable Nitrogen Isotope Analysis of Amino Acid Enantiomers by Gas Chromatography/Combustion/Isotope Ratio Mass Spectrometry作者:Stephen A. Macko、Maria E. Uhle、Michael H. Engel、Vladimir AndrusevichDOI:10.1021/ac960956l日期:1997.3.1analysis. Following hydrolysis and derivatization, single-component isotope analysis is accomplished on nanomole quantities of each of the stereoisomers of an amino acid, utilizing the effluent stream of gas chromatographic separation. Nitrogen isotope fractionation is minimal during acylation of the amino acid, with no additional nitrogen being added stoichiometrically to the derivative. Thus, the isotopic提出了通过使用气相色谱/燃烧/同位素比质谱法(GC / C / IRMS)分析单个氨基酸立体异构体的稳定氮同位素组成的方法。单个氨基酸或其对映异构体的氮同位素组成可能不需要常规稳定同位素分析所需的劳动强度大且耗时的制备规模的色谱程序。在水解和衍生化之后,利用气相色谱分离的流出物流,对纳摩尔量的每种氨基酸立体异构体进行单组分同位素分析。在氨基酸酰化过程中,氮同位素的分馏极少,没有化学计量地将额外的氮添加到衍生物中。因此,衍生物中氮的同位素组成与原始化合物相同。通过常规同位素比质谱法(IRMS)和GC / C / IRMS对11个氨基酸及其三氟乙酰基(TFA)/异丙基(IP)酯衍生物进行了重复的稳定氮同位素分析,结果表明,气相色谱程序可高度重现(标准偏差通常为每千分之0.3-0.4),而氨基酸与其TFA / IP衍生物之间的同位素差异通常小于每千分之0.5。
表征谱图
-
氢谱1HNMR
-
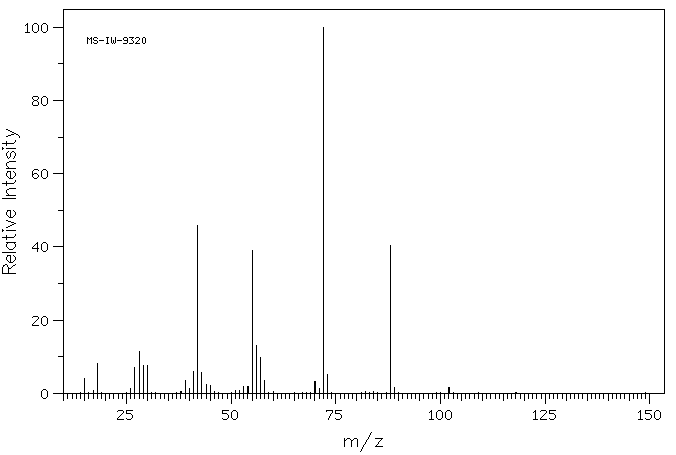
质谱MS
-
碳谱13CNMR
-
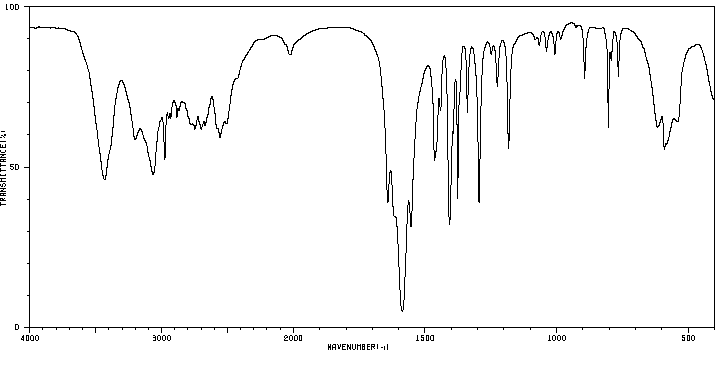
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸