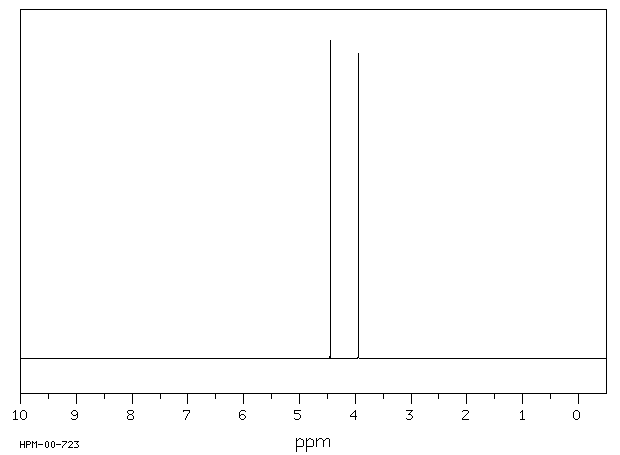
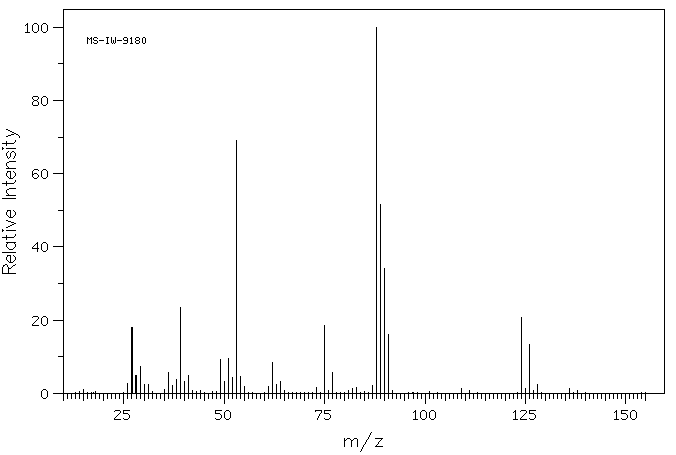
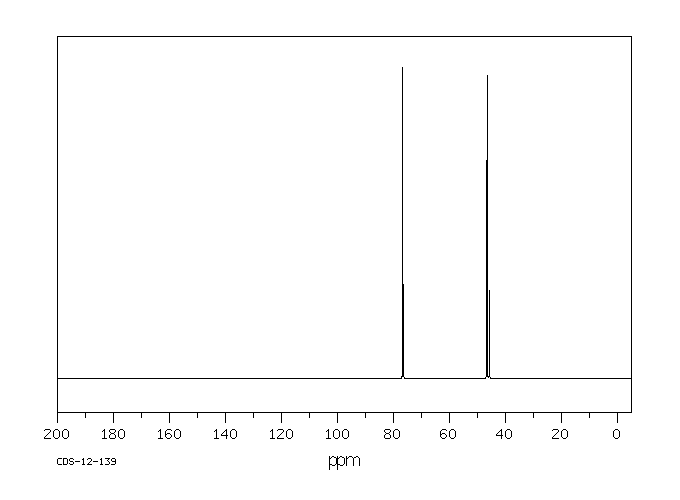
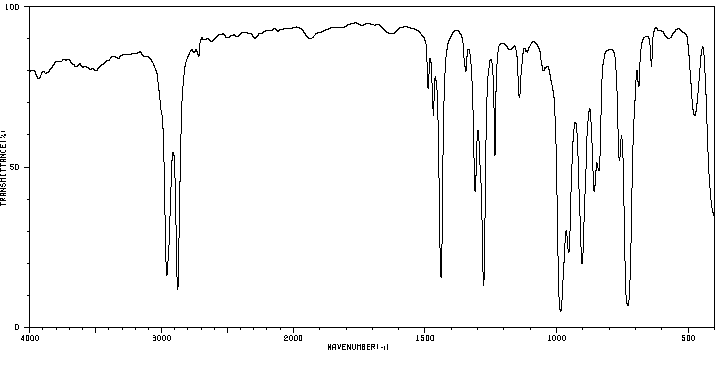
毒理性
神经毒素 - 其他中枢神经系统神经毒素
肾毒素 - 该化学物质在工作环境中可能对肾脏有毒。
催泪剂(催泪瓦斯)- 刺激眼睛并引起流泪的物质。
Neurotoxin - Other CNS neurotoxin
Nephrotoxin - The chemical is potentially toxic to the kidneys in the occupational setting.
Lacrimator (Lachrymator) - A substance that irritates the eyes and induces the flow of tears.
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases