4-孕烯-3,11,20-三酮 | 516-15-4
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:170-175 °C(lit.)
-
沸点:487.6±45.0 °C(Predicted)
-
密度:1.14±0.1 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少量)、乙酸乙酯(少量)
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:24
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.76
-
拓扑面积:51.2
-
氢给体数:0
-
氢受体数:3
安全信息
-
RTECS号:TU5513000
-
储存条件:储存于阴凉、干燥、通风良好的库房。远离火种和热源,防止阳光直射,并确保包装密封。应与酸类及食用化学品分开存放,切忌混储。库区应备有合适的材料以收容泄漏物。
SDS
: Pregn-4-ene-3,11,20-trione
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H301 吞咽会中毒
警告申明
预防
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
措施
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P330 漱口。
储存
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C21H28O3
分子式
: 328.45 g/mol
分子量
组分 浓度或浓度范围
Pregn-4-ene-3,11,20-trione
-
CAS 号 516-15-4
EC-编号 208-222-1
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
戴呼吸罩。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 将人员撤离到安全区域。
避免吸入粉尘。
6.2 环境保护措施
在确保安全的前提下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息以前和操作过此产品之后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N99型(US)
或P2型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
辛醇--水的分配系数的对数值: 2.206
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
细胞突变性-体外试验 - 人 - 淋巴细胞
DNA抑制
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
致畸性 - 兔子 - 未报道的
对生殖的影响:胚胎植入前死亡率(例如每个雌性的植入胚胎数减少;每个黄体的植入总数。
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: TU5513000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2811 国际海运危规: 2811 国际空运危规: 2811
14.2 联合国(UN)规定的名称
欧洲陆运危规: TOXIC SOLID, ORGANIC, N.O.S. (Pregn-4-ene-3,11,20-trione)
国际海运危规: TOXIC SOLID, ORGANIC, N.O.S. (Pregn-4-ene-3,11,20-trione)
国际空运危规: Toxic solid, organic, n.o.s. (Pregn-4-ene-3,11,20-trione)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
暂无相关信息。
用途暂无相关信息。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 11-去氢皮质甾酮 11-dehydrocorticosterone 72-23-1 C21H28O4 344.451 —— progesterone 57-83-0 C21H30O2 314.468 11Alpha-孕酮 11-alpha-hydroxyprogesterone 80-75-1 C21H30O3 330.467 11B-羟孕酮 (8S,9S,10R,11S,13S,14S,17S)-17-Acetyl-11-hydroxy-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one 600-57-7 C21H30O3 330.467 肾上腺酮 Corticosterone 50-22-6 C21H30O4 346.467 可的松 Cortisone 53-06-5 C21H28O5 360.45 —— 9α-bromo-11β-fluoroprogesterone 4031-30-5 C21H28BrFO2 411.355 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 11-去氢皮质甾酮 11-dehydrocorticosterone 72-23-1 C21H28O4 344.451 [2-[(8S,9S,10R,13S,14S,17S)-10,13-二甲基-3,11-二氧代-2,6,7,8,9,12,14,15,16,17-十氢-1H-环戊并[a]菲-17-基]-2-氧代乙基]乙酸酯 21-acetoxy-pregn-4-ene-3,11,20-trione 1173-27-9 C23H30O5 386.488 孕甾-1,4-二烯-3,11,20-三酮 Pregn-1,4-diene-3,11,20-trione 4368-11-0 C21H26O3 326.436 —— 3,11,20-Trioxo-pregnadien-4,6 21063-91-2 C21H26O3 326.436 —— 6β-hydroxypregn-4-ene-3,11,20-trione 28449-15-2 C21H28O4 344.451 11B-羟孕酮 (8S,9S,10R,11S,13S,14S,17S)-17-Acetyl-11-hydroxy-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-cyclopenta[a]phenanthren-3-one 600-57-7 C21H30O3 330.467 —— 11β-hydroxypregnenolone 1164-86-9 C21H32O3 332.483 —— 17-acetyl-10,13-dimethyl-1,6,7,8,9,10,12,13,14,15,16,17-dodecahydro-2H-cyclopenta[a]phenanthrene-3,11-dione-3,21-diethylene ketal 2302-12-7 C25H36O5 416.558
反应信息
-
作为反应物:描述:4-孕烯-3,11,20-三酮 在 2,3,4,5,6-pentahydroxy-hexanal 、 palladium 10% on activated carbon 、 氢气 、 nicotinamide adenine dinucleotide 、 3α-hydroxysteroid dehydrogenase from Comamonas testosteroni 、 GDH from Bacillus subtilis QB928 、 sodium hydroxide 作用下, 以 四氢呋喃 、 水 、 叔丁醇 为溶剂, 25.0~30.0 ℃ 、13.79 kPa 条件下, 生成 阿法沙龙参考文献:名称:羟基类固醇脱氢酶催化类固醇中 3-酮的高度区域选择性、化学选择性和对映选择性氢化摘要:证明了在羟基类固醇脱氢酶 (HSDH) 的催化下,类固醇中的 3-酮基可以高度选择性地氢化为 3-羟基类固醇。 Ct3α-HSDH催化的氢化反应以高产率产生了作为主要对映体纯异构体的3α-羟基类固醇,而Ss3β-HSDH催化体系则以优异的产率产生了3β-羟基类固醇。在两种催化体系中,氢化反应在 3-酮基上进行区域选择性,7-、11-、17-和 20-酮基几乎未反应,并且在 C=C 键和酯基未受攻击的情况下进行化学选择性氢化。我们的HSDH促进的氢化反应具有区域选择性、化学选择性和对映选择性高、收率好、条件温和、底物范围广、适合克级合成等优点。值得注意的是,通过我们的氢化方法,可以轻松、高产地获得脱氢表雄酮、布烯醇酮和阿法沙酮等生物活性分子。DOI:10.1021/acs.orglett.3c03557
-
作为产物:描述:参考文献:名称:Microbiological Transformations of Steroids.1 I. Introduction of Oxygen at Carbon-11 of Progesterone摘要:DOI:10.1021/ja01143a033
文献信息
-
GLUTATHIONE-CHOLESTEROL DERIVATIVES AS BRAIN TARGETING AGENTS申请人:South Dakota Board of Regents公开号:US20200048305A1公开(公告)日:2020-02-13The present invention describes compositions containing cholesterol-linker-glutathione conjugates for targeting the brain by overcoming barrier entry to the CNS through the blood brain barrier (BBB), including micelle and liposome forms of such compositions. In addition, methods for treating subjects by administering such compositions are also disclosed.
-
Pyrazolones as inhibitors of 11B-hydroxysteroid dehydrogenase申请人:Banner Lester Bruce公开号:US20070049632A1公开(公告)日:2007-03-01Provided herein are compounds of the formula (I): as well as pharmaceutically acceptable salts thereof, wherein the substituents are as those disclosed in the specification. These compounds, and the pharmaceutical compositions containing them, are useful for the treatment of diseases such as, for example, type II diabetes mellitus and metabolic syndrome.本文提供了以下式(I)的化合物: 以及其药学上可接受的盐,其中取代基如规范中所披露的那样。这些化合物及含有它们的药物组合物对于治疗诸如II型糖尿病和代谢综合征等疾病是有用的。
-
Microbial hydroxylation of steroids. 8. Incubation of Cn halo- and other substituted steroids with Cn hydroxylating fungi作者:Herbert L. Holland、Everton M. ThomasDOI:10.1139/v82-027日期:1982.1.15
A series of C-21 substituted progesterone derivatives (R = F, Cl, Br, CH3) has been prepared, and incubated the C-21 hydroxylating fungus A. niger. No biotransformation was observed where R = Cl, Br, or CH3, but a minor amount of hydroxylation occurred at an unidentified site where R = F. Two C-11β substituted progesterone derivatives (R = F, OH) were converted to the corresponding C-11 ketone by the C-11α hydroxylator R. stolonifer, but a C-11β hydroxylator (C. lunata) was unable to perform a similar transformation with a C-11α hydroxy substrate. C. lunata hydroxylated a C-11β fluoro substrate at C-14α and C-21 in low yield. C-7α and -7β-hydroxy androst-4-ene-3,17-diones were prepared and incubated with C-7β hydroxylating (R. stolonifer) and C-7α hydroxylating (M. griseocyanus) fungi respectively. No ketone formation was observed. Similarly, the C-6β hydroxylator R. arrhizus was unable to transform a C-6α-hydroxy substrate or C-6α- or C-6β-hydroxy-B-norsteroids. No oxidation at halogen was observed for any of the substrates used. 13C–19F coupling constants for 11β-fluoroprogesterone suggest the presence of through space interactions between fluorine and both C-18 and C-19.
一系列C-21取代孕酮衍生物(R = F、Cl、Br、CH3)已经制备,并与C-21羟化真菌A. niger培养。观察到当R = Cl、Br或 时未发生生物转化,但当R = F时,在未知位置发生了少量羟基化。两种C-11β取代孕酮衍生物(R = F、OH)被C-11α羟化酶R. stolonifer转化为相应的C-11酮,但C-11β羟化酶(C. lunata)无法使用C-11α羟基底物进行类似转化。C. lunata在低产率下将C-11β氟底物羟化为C-14α和C-21。制备了C-7α和-7β-羟基雄烯-4-烯-3,17-二酮,并分别与C-7β羟化(R. stolonifer)和C-7α羟化(M. griseocyanus)真菌培养。未观察到酮形成。同样,C-6β羟化酶R. arrhizus无法转化C-6α-羟基底物或C-6α-或C-6β-羟基B-诺甾醇。未观察到任何底物的卤素氧化。11β-氟孕酮的13C–19F偶合常数表明氟与C-18和C-19之间存在空间相互作用。 -
ミトコンドリア生合成ならびにミトコンドリア機能不全または欠乏に関連する疾患において使用するための11β−ヒドロキシステロイドの新規の化合物申请人:スファエラ ファーマ プライベート リミテッド公开号:JP2016505038A公开(公告)日:2016-02-18本発明は、11β−ヒドロキシステロイドの新規の化合物および組成物、ならびにヒトまたは動物対象におけるミトコンドリアの損傷を予防または逆転するための医薬品、ミトコンドリア機能不全または欠乏に関連する疾患を処置または予防するための医薬品、およびミトコンドリア構造および機能の異常に関連する疾患を処置する手段としてミトコンドリアの再生または再構成を誘導するための医薬品としてのそれらの適用を提供する。また、本明細書において、ミトコンドリアの損傷を診断する方法、およびミトコンドリアの損傷また欠乏を処置、予防、または逆転するように設計された治療薬の成功または失敗を診断するための方法を開示する。【選択図】なし这项发明提供了一种新的11β-羟基类固醇化合物和组合物,以及用于预防或逆转人类或动物体内线粒体损伤的药物,用于治疗或预防线粒体功能不全或缺乏相关疾病的药物,以及作为治疗线粒体结构和功能异常相关疾病的手段,诱导线粒体再生或重建的药物的应用。此外,本说明书揭示了一种诊断线粒体损伤的方法,以及一种诊断治疗药物成功或失败的方法,该治疗药物设计用于治疗线粒体损伤或缺乏、预防或逆转。【选择图】无
-
Aryl sulfonyl piperidines申请人:Gillespie Paul公开号:US20060199816A1公开(公告)日:2006-09-07Provided herein are compounds of the formula (1): as well as pharmaceutically acceptable salts thereof, wherein the substituents are as those disclosed in the specification. These compounds, and the pharmaceutical compositions containing them, are useful for the treatment of diseases such as, for example, type II diabetes mellitus and metabolic syndrome.本文提供了以下式(1)的化合物: 以及其药学上可接受的盐,其中取代基如规范中所披露的那样。这些化合物及含有它们的药物组合物对于治疗诸如II型糖尿病和代谢综合征等疾病是有用的。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
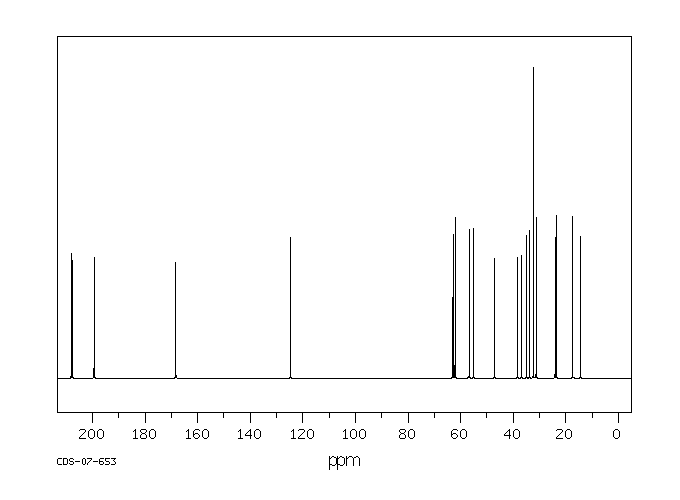
碳谱13CNMR
-
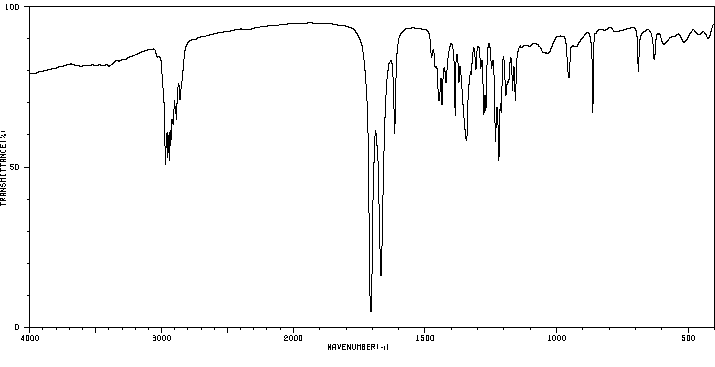
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息