玫瑰红酸二水合物 | 118-76-3
中文名称
玫瑰红酸二水合物
中文别名
玫琮酸;玫琮酸二水合物;5,6-二羥-5-環己烯四酮;玫棕酸
英文名称
rhodizonic acid
英文别名
Rhodizonsaeure;5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone
CAS
118-76-3
化学式
C6H2O6
mdl
MFCD00001651
分子量
170.078
InChiKey
WCJLIWFWHPOTAC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:248 °C (dec.)(lit.)
-
沸点:259.67°C (rough estimate)
-
密度:1.7636 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):-1
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:109
-
氢给体数:2
-
氢受体数:6
安全信息
-
安全说明:S24/25
-
海关编码:2914400090
SDS
| Name: | Rhodizonic Acid Dihydrate Material Safety Data Sheet |
| Synonym: | None. |
| CAS: | 118-76-3 |
Synonym: None.
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 118-76-3 | Rhodizonic Acid Dihydrate | ca 100 | 204-276-5 |
Risk Phrases: None Listed.
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW The toxicological properties of this material have not been fully investigated. Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
SECTION 4 - FIRST AID MEASURES
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation.
SECTION 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 118-76-3: Personal Protective Equipment
Eyes:
Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Decomposes
Freezing/Melting Point: 255.00 - 257.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: > 257 deg C
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H2O6.2H2O
Molecular Weight: 206.11
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 118-76-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Rhodizonic Acid Dihydrate - Not listed by ACGIH, IARC, or NTP.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material.
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases: S 24/25 Avoid contact with skin and eyes. S 28A After contact with skin, wash immediately with plenty of water. S 37 Wear suitable gloves. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 118-76-3: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 118-76-3 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 118-76-3 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 2/05/1999 Revision #2 Date: 3/18/2003 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 巴豆酸 croconic acid 488-86-8 C5H2O5 142.068
反应信息
-
作为反应物:参考文献:名称:μ-Rhodizonato-1κO,1:2κO',2κO''-tetra(triphenylphosphine)disilver(I):具有[C6O6]2-配体模板的分子复合物摘要:呈现了第一种玫瑰红酸银和第四种过渡金属玫瑰红酸配合物。标题化合物显示了迄今为止未观察到的玫瑰红配体的配位模式,该模式因平面性而异样地扭曲。结构讨论伴随着对迄今为止在结构上表征的玫瑰红盐和配合物的全面文献综述。DOI:10.1002/zaac.201500589
-
作为产物:参考文献:名称:13 C-NMR。环状碳氧化合物的光谱,结构和反应性† ‡摘要:通过13 C-NMR研究了在不同溶剂中环状聚酮的结构,它们的水合物和还原产物。光谱学。在二甲亚砜溶液中,Rhodizonic acid (1),croconic acid (2)和方酸(3)显示信号平均。在无水四氢呋喃1和2中可以观察到非动态物种。十二羟基环己烷(5)和十羟基环戊烷('leuconic酸')(6)的自发反应并研究了环缩产物的形成顺序。可以清楚地识别出关键的中间体,这些中间体支持了先前提出的机制,该机制涉及苯甲酸类型的重排,然后进行脱羧和随后的氧化还原反应。DOI:10.1002/hlca.19770600325
-
作为试剂:参考文献:名称:在存在Rhodizonic acid的情况下,通过还原的烟酰胺腺嘌呤二核苷酸模型还原苯甲酰基甲酸甲酯摘要:在室温下用1-苄基-1,4-二氢烟碱酰胺(BNAH; NADH模型)还原Rhodizonic acid(RA),得到四羟基对醌(THQ)和六羟基苯(HHB)。二电子还原和四电子还原。在存在RA的情况下,用BNAH进行的苯甲酰甲酸酯的还原反应进行得很顺利,但没有RA则无法完全还原。DOI:10.1016/s0040-4039(00)84055-1
文献信息
-
Luminescent compounds申请人:——公开号:US20030235846A1公开(公告)日:2003-12-25Luminescent compounds, reactive intermediates used to synthesize luminescent compounds, and methods of synthesizing and using luminescent compounds. These compounds may be based on squaric, croconic, and rhodizonic acid, and their analogs, among others, and relate generally to the structure: 1 where Z is a four, five, or six-member aromatic ring, and A, B, C, D, E, and F are substituents of Z, in any order, that may include O, S, Se, Te, C(R a )(R b ), N-R c , N(R d )(R e ), W 1 , and W 2 . Generally, each compound includes at least one of W 1 or W 2 , where W 1 and W 2 have the structures: 2 respectively. The compounds may include a reactive group and/or a carrier. The luminescent compounds may be useful in both free and conjugated forms as probes, labels, and/or indicators.发光化合物,用于合成发光化合物的反应中间体,以及合成和使用发光化合物的方法。这些化合物可能基于方酰、克罗酰和罗地酮酸及其类似物等,通常与结构相关:其中Z是一个四、五或六元芳香环,而A、B、C、D、E和F是Z的取代基,可以包括O、S、Se、Te、C(Ra)(Rb)、N-Rc、N(Rd)(Re)、W1和W2,顺序任意。通常,每种化合物至少包括W1或W2中的一种,其中W1和W2的结构分别为:。这些化合物可能包括一个反应基团和/或一个载体。这些发光化合物可能在自由和共轭形式下作为探针、标记物和/或指示剂而有用。
-
Insertion von Rhodizonsäure in die Gallium-Gallium- bzw. Indium-Indium-Bindungen von Digallan(4)- bzw. Diindan(4)-Verbindungen作者:Werner Uhl、Malte PröttDOI:10.1002/1521-3749(200211)628:11<2259::aid-zaac2259>3.0.co;2-c日期:2002.11l] digallan(4) (1) und der entsprechenden Diindiumverbindung (2) unter Oxidation der Gallium- bzw. Indiumatome von +II nach +III. Ein Protonentransfer zu den elementorganischen Gruppen findet nicht statt, vielmehr insertiert sich die Saure vollstandig in die Element-Element-Bindungen. Die Dialkylgallium- bzw. Dialkylindiumreste der Produkte (3 (Ga) und 4 (In)) werden durch jeweils zwei SauerstoffatomeRhodizonsaure (C6O6H2, 5, 6-Dihydroxy-5-cyclohexen-1, 2, 3, 4-tetraon) reagiert mit Tetrakis[bis(trimethylsilyl)-methyl] digallan(4) (1) und der entsprechenden Diindiumverbindung (2) unter氧化 der 镓 - bzw。Indiumatome von +II nach +III。Ein Protonentransfer zu den elementorganischen Gruppen findet nicht statt, vielmehr insertiert sich die Saure vollstandig in die Element-Element-Bindungen。模具二烷基镓-bzw。Dialkylindiumreste
-
Smectic liquid crystals based on hexaazatriphenylene: potential organic n-type semiconductor作者:Baoxiang Gao、Licui Zhang、Qianqian Bai、Ying Li、Junwei Yang、Lixiang WangDOI:10.1039/c0nj00586j日期:——We report the synthesis of dibenzohexaazatriphenylene derivative DBHAT. The addition of electron-withdrawing groups on the dibenzohexaazatriphenylene core leads to a high electron-accepting capability. DBHAT exhibits reversible four-step reduction waves at half-wave potentials (E1/2) of −0.42, −0.94, −1.35 and −1.80 V vs. Ag/AgCl. Furthermore, DBHAT is able to form a smectic liquid crystalline assembly over a wide temperature range. With strong dipole–dipole interactions, the smectic liquid crystals show extremely highly stabilized mesophases.
-
Synthesis and Optical Properties of Unsymmetrically Substituted Triarylamine Hexaazatrinaphthalenes作者:Christopher B. Larsen、Jonathan E. Barnsley、Holly van der Salm、Michael G. Fraser、Nigel T. Lucas、Keith C. GordonDOI:10.1002/ejoc.201700251日期:2017.5.3We report herein the synthesis and optical properties of unsymmetrically substituted hexaazatrinaphthalene (HATN) systems, and the influence of donor additivity on the electronic properties of the material. The hexaazatrinaphthalenes have been studied by means of electrochemical, electronic absorption and emission, FT Raman and resonance Raman methodologies coupled with DFT calculations. The optical
-
Reversible solid-state interconversion of rhodizonic acid H<sub>2</sub>C<sub>6</sub>O<sub>6</sub>into H<sub>6</sub>C<sub>6</sub>O<sub>8</sub>and the solid-state structure of the rhodizonate dianion C<sub>6</sub>O<sub>6</sub><sup>2−</sup>(aromatic or non-aromatic?)作者:Dario Braga、Gianna Cojazzi、Lucia Maini、Fabrizia GrepioniDOI:10.1039/b107317f日期:——Anhydrous rhodizonic acid H2C6O6 is obtained by (fully reversible) thermal dehydration of solid 2,3,5,5,6,6-hexahydroxycyclohex-2-ene-1,4-dione, H6C6O8, commonly known as rhodizonic acid dihydrate. Treatment of H6C6O8 with RbOH yields crystals of Rb2C6O6; the oxocarbon dianion C6O62− is shown to possess a flat, benzene-type structure, with C–C bonds shorter than expected for a (non-aromatic) ketonic-type
表征谱图
-
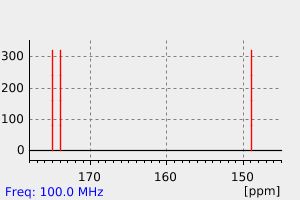
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
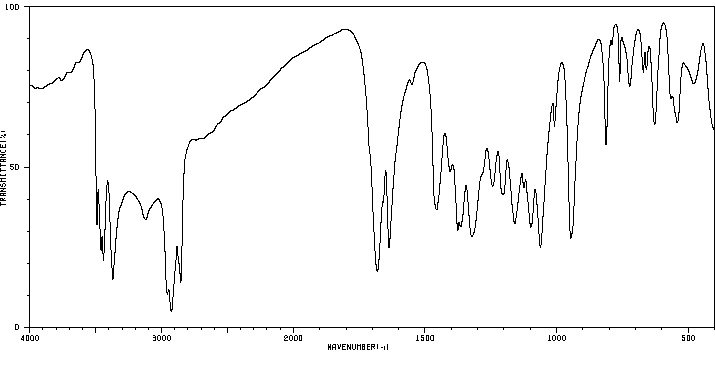
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷