5-甲基-十一烷 | 1632-70-8
中文名称
5-甲基-十一烷
中文别名
——
英文名称
5-methylundecane
英文别名
5-Methylundecan
CAS
1632-70-8
化学式
C12H26
mdl
——
分子量
170.338
InChiKey
QULNVKABFWNUCW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-50.8°C (estimate)
-
沸点:206°C
-
密度:0.7476
-
保留指数:1156;1157.14;1157.24;1157.29;1159;1150;1152;1152;1156;1157;1158;1158;1158;1154;1156;1157.4
计算性质
-
辛醇/水分配系数(LogP):6.4
-
重原子数:12
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为产物:参考文献:名称:Reaction of Raney nickel with alcohols摘要:DOI:10.1021/jo00249a007
文献信息
-
Formation of Three-Membered Rings by S<sub>H</sub>i Displacement. Reverse of Cyclopropyl Ring Opening<sup>1</sup>作者:Dennis D. Tanner、Liying Zhang、Li Qing Hu、Pramod KandanarachchiDOI:10.1021/jo960879u日期:1996.1.1carried out in the presence of several transfer agents (CCl(4), CCl(3)Br, CCl(2)Br(2)) initiate a radical chain addition of CCl(2)X(*) and yield cyclized materials resulting from the S(H)i displacement of halogen by a carbon-centered radical. The radical displacement of a halogen on carbon, the reverse of homolytic displacement on cyclopropyl carbon, is dominant at low temperatures. The rate constants for通常的方法是将卤甲烷光引发或过氧化物引发的自由基甲烷加成烯烃,在20至100摄氏度的温度范围内产生1,2-加成产物。在较低的温度(-42至-104摄氏度)下,竞争性反应加入CCl(2)X(*)之后,生成烷基环丙烷。1-辛烯或1-己烯和1-甲基环己烯与氢原子的反应在几种转移剂(CCl(4),CCl(3)Br,CCl(2)Br(2))的存在下进行CCl(2)X(*)的链加成并产生环化的材料,该环化的材料是由碳中心自由基取代卤素的S(H)i。在低温下,卤素在碳上的自由基置换与环丙基碳上的均质置换相反。环化速率常数(k(c))与卤代甲烷转移速率常数(k(t))的等速动力学温度为-46摄氏度(CCl(4),1-己烯);-35摄氏度(CCl(4),1-甲基环己烯)。在BrCCl(3)存在下进行的两种底物反应的等动力学温度计算为-204摄氏度(1-辛烯)和-109摄氏度(1-甲基环己烯)。
-
COSMETIC EXCIPIENT INCLUDING A C8-C10 ALKANE AND A C>= 11 ALKANES申请人:BIOSYNTHIS公开号:US20210154110A1公开(公告)日:2021-05-27A cosmetic excipient including an alkane mixture, the alkane mixture including at least one C8-C10 alkane, and at least one C≥11 alkane, wherein the at least one C8-C10 alkane is present in a percentage by mass less than or equal to approximately 40% (≤40%), relative to the total mass of the alkane mixture.
-
Synthesis, characterization and isomerization performance of micro/mesoporous materials based on H-ZSM-22 zeolite作者:Suyao Liu、Jie Ren、Huaike Zhang、Enjing Lv、Yong Yang、Yong-Wang LiDOI:10.1016/j.jcat.2015.12.009日期:2016.3in alkaline solution with cetyltrimethylammonium bromide template (CTAB). The structure, morphology, pore properties, acidity and isomerization performance of the catalysts by using the resulting materials were characterized and assessed. The dissolution and recrystallization procedure introduced the well-developed mesoporous structure of MCM-41 type with the meso-scale channels of about 3 nm in size通过用十六烷基三甲基溴化铵模板(CTAB)在碱性溶液中重结晶H-ZSM-22沸石,制备具有不同介孔率的微/介孔材料。通过使用所得材料对催化剂的结构,形态,孔性能,酸度和异构化性能进行了表征和评估。溶解和重结晶过程引入了发达的MCM-41型介孔结构,该介孔结构在H-ZSM-22微孔沸石的外表面上形成了尺寸约为3 nm的介孔通道,形成了微孔/介孔材料,其在孔口具有增加的弱B酸位点和减少的总酸位点数量。结果表明,发达的中孔的存在可以显着提高对多支链产物的选择性,并抑制正十二烷异构化过程中的侧裂反应。与原始的微孔Pt / H-ZSM-22催化剂相比,具有合适重结晶度的微孔/中孔Pt / ZSM-22 / MCM-41双功能催化剂在长链正构烷烃异构化过程中,在高转化率下具有高异构化选择性。
-
Copper-catalyzed coupling reaction of unactivated secondary alkyl iodides with alkyl Grignard reagents in the presence of 1,3-butadiene as an effective additive作者:Ruwei Shen、Takanori Iwasaki、Jun Terao、Nobuaki KambeDOI:10.1039/c2cc34847k日期:——Cu-catalyzed cross-coupling of unactivated secondary alkyl iodides with alkyl Grignard reagents in the presence of 1,3-butadiene as a ligand precursor was developed. The use of 1,3-butadiene resulted in improved yields of alkyl-alkyl products with improved selectivities.
-
1-Hexene: a renewable C6 platform for full-performance jet and diesel fuels作者:Benjamin G. Harvey、Heather A. MeylemansDOI:10.1039/c3gc41554f日期:——A highly efficient and selective process has been developed for the conversion of 1-hexene to jet and diesel fuels. In combination with commercial processes for the dehydration of ethanol and trimerization of ethylene, this work provides a basis for the synthesis of full-performance hydrocarbon fuels from bio-ethanol. Selective oligomerization of 1-hexene with a Cp2ZrCl2/MAO catalyst at ambient temperature and pressure resulted in 100% conversion of 1-hexene with >80% selectivity to a mixture of the dimer and trimer. The hydrogenated dimer had a −20 °C viscosity of only 3.5 mPa s, an exceptionally low freezing point of −77 °C, and a cetane number of 67 suggesting that it has performance characteristics suitable for both jet and diesel fuels. The hydrogenated trimer had a flash point of 128 °C, a cetane number of 92, a 40 °C viscosity of 3.1 mPa s, and a −20 °C viscosity of 24.5 mPa s. These properties suggest that the trimer has applications as a high-performance diesel fuel. In addition to the fuel-range hydrocarbons, heavier oligomers have potential as biolubricants which improves the carbon yield of useful products to near quantitative levels.我们开发出了一种将 1-己烯转化为喷气燃料和柴油燃料的高效选择性工艺。结合乙醇脱水和乙烯三聚的商业工艺,这项工作为利用生物乙醇合成高性能碳氢化合物燃料奠定了基础。在常温常压下,使用 Cp2ZrCl2/MAO 催化剂对 1-hexene 进行选择性低聚,可将 1-hexene 100%转化为二聚体和三聚体的混合物,选择性大于 80%。氢化二聚体的 -20 °C 粘度仅为 3.5 mPa s,凝固点特别低,为 -77 °C ,十六烷值为 67,这表明它具有适用于喷气燃料和柴油燃料的性能特点。氢化三聚体的闪点为 128 °C,十六烷值为 92,40 °C 粘度为 3.1 mPa s,-20 °C 粘度为 24.5 mPa s。除了燃料范围内的碳氢化合物外,较重的低聚物还有可能用作生物润滑剂,从而将有用产品的碳产量提高到接近定量水平。
表征谱图
-
氢谱1HNMR
-
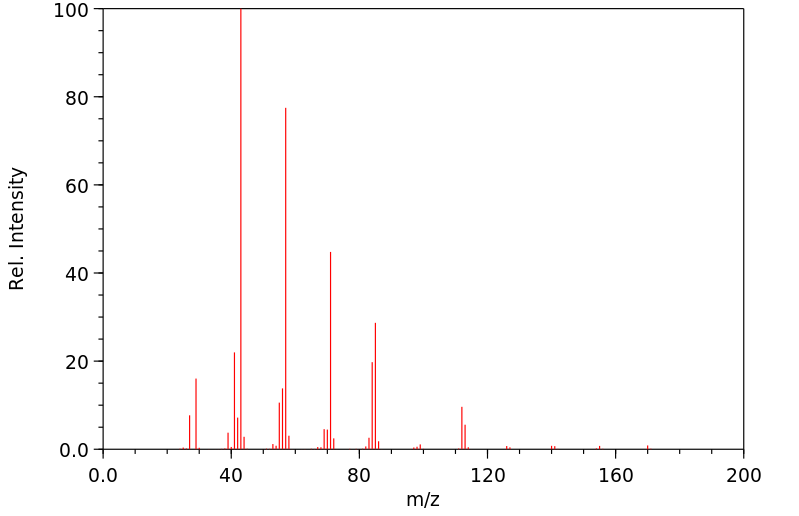
质谱MS
-
碳谱13CNMR
-
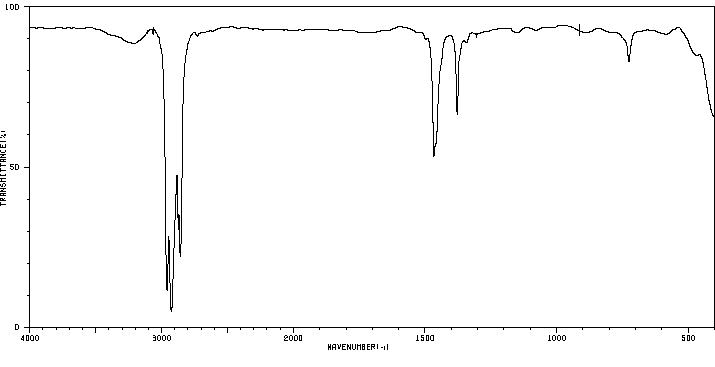
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷