2-(丙氨基)乙醇 | 16369-21-4
物质功能分类
中文名称
2-(丙氨基)乙醇
中文别名
2-(丙基氨基)乙醇
英文名称
N-(2-hydroxyethyl)-N-propylamine
英文别名
2-(Propylamino)ethanol;2-(propylamino)ethan-1-ol
CAS
16369-21-4
化学式
C5H13NO
mdl
MFCD00002844
分子量
103.164
InChiKey
BCLSJHWBDUYDTR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:29.84°C (estimate)
-
沸点:182 °C (lit.)
-
密度:0.9 g/mL at 25 °C (lit.)
-
闪点:178 °F
-
稳定性/保质期:
按规定使用该产品不会分解,并应注意避免与氧化剂、热源及明火接触。
计算性质
-
辛醇/水分配系数(LogP):0
-
重原子数:7
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:32.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:C
-
危险类别码:R20/21/22,R34
-
危险品运输编号:UN 2735 8/PG 2
-
WGK Germany:3
-
RTECS号:KM2885000
-
海关编码:2922199090
-
安全说明:S26,S36/37/39,S45
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H317,H319
-
储存条件:存于密闭、阴凉、干燥处。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 2-(Propylamino)ethanol
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 16369-21-4
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Inhalation (Category 4), H332
Acute toxicity, Dermal (Category 4), H312
Acute toxicity, Oral (Category 4), H302
Skin corrosion (Category 1B), H314
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
C Corrosive R20/21/22, R34
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
H302 Harmful if swallowed.
H312 Harmful in contact with skin.
H314 Causes severe skin burns and eye damage.
H332 Harmful if inhaled.
Precautionary statement(s)
P280 Wear protective gloves/ protective clothing/ eye protection/ face
protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
P310 Immediately call a POISON CENTER or doctor/ physician.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Synonyms : N-Propylethanolamine
Formula : C5H13NO
Molecular Weight : 103,16 g/mol
CAS-No. : 16369-21-4
EC-No. : 240-426-6
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
2-(Propylamino)ethanol
CAS-No. 16369-21-4 Acute Tox. 4; Skin Corr. 1B; <= 100 %
EC-No. 240-426-6 H302, H312, H314, H332
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
2-(Propylamino)ethanol
CAS-No. 16369-21-4 C, R20/21/22 - R34 <= 100 %
EC-No. 240-426-6
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Take off contaminated clothing and shoes immediately. Wash off with soap and plenty of water. Consult a
physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
For small (incipient) fires, use media such as "alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large quantities (flooding) of water applied as a mist or
spray; solid streams of water may be ineffective. Cool all affected containers with flooding quantities of
water.
Special hazards arising from the substance or mixture
Carbon oxides, nitrogen oxides (NOx)
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapours, mist or gas. Ensure adequate ventilation.
Remove all sources of ignition. Evacuate personnel to safe areas. Beware of vapours accumulating to
form explosive concentrations. Vapours can accumulate in low areas.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13). Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
Apart from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Tightly fitting safety goggles. Faceshield (8-inch minimum). Use equipment for eye protection
tested and approved under appropriate government standards such as NIOSH (US) or EN
166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: colourless
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 182 °C - lit.
boiling range
g) Flash point 78 °C - closed cup
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 0,9 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks.
Incompatible materials
Strong oxidizing agentsStrong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
LD50 Dermal - rat - 1.797 mg/kg
Remarks: Sense Organs and Special Senses (Nose, Eye, Ear, and Taste):Eye:Chromodacryorrhea.
Behavioral:Tremor. Prolonged skin contact may cause skin irritation and/or dermatitis.
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: KM2885000
Material is extremely destructive to tissue of the mucous membranes and upper respiratory tract, eyes, and
skin., spasm, inflammation and edema of the larynx, spasm, inflammation and edema of the bronchi,
pneumonitis, pulmonary edema, burning sensation, Cough, wheezing, laryngitis, Shortness of breath,
Headache, Nausea
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
This combustible material may be burned in a chemical incinerator equipped with an afterburner and
scrubber. Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: 2735 IMDG: 2735 IATA: 2735
UN proper shipping name
ADR/RID: AMINES, LIQUID, CORROSIVE, N.O.S. (2-(Propylamino)ethanol)
IMDG: AMINES, LIQUID, CORROSIVE, N.O.S. (2-(Propylamino)ethanol)
IATA: Amines, liquid, corrosive, n.o.s. (2-(Propylamino)ethanol)
Transport hazard class(es)
ADR/RID: 8 IMDG: 8 IATA: 8
Packaging group
ADR/RID: II IMDG: II IATA: II
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Full text of H-Statements referred to under sections 2 and 3.
Acute Tox. Acute toxicity
H302 Harmful if swallowed.
H312 Harmful in contact with skin.
H314 Causes severe skin burns and eye damage.
H332 Harmful if inhaled.
Skin Corr. Skin corrosion
Full text of R-phrases referred to under sections 2 and 3
C Corrosive
R20/21/22 Harmful by inhalation, in contact with skin and if swallowed.
R34 Causes burns.
Further information
Copyright 2014 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
反应信息
-
作为反应物:描述:参考文献:名称:Histamine Antagonists. VII. Phenothiazine Derivatives1摘要:DOI:10.1021/ja01164a060
-
作为产物:描述:2-vinyloxyethylpropylamine 在 硫酸 作用下, 反应 3.0h, 以52%的产率得到2-(丙氨基)乙醇参考文献:名称:Synthesis of new 3-substituted 1,3-oxazolidine-2-thiones摘要:Reaction of N-alkylsubstituted ethanolamines with carbon disulfide followed by reaction with benzoyl chloride afforded N-substituted oxazolidine-2-thiones.DOI:10.1134/s1070363217060299
文献信息
-
[EN] PHOSPHONOOXY QUINAZOLINE DERIVATIVES AND THEIR PHARMACEUTICAL USE<br/>[FR] DERIVES DE PHOSPHONOOXY QUINAZOLINE ET LEUR UTILISATION PHARMACEUTIQUE申请人:ASTRAZENECA AB公开号:WO2004058781A1公开(公告)日:2004-07-15Quinazoline derivatives of formula (I) wherein A is 5-membered heteroaryl containing a nitrogen atom and one or two further nitrogen atoms; compositions containing them, processes for their preparation and their use in therapy.
-
Structural Requirements for Binding of Anandamide-Type Compounds to the Brain Cannabinoid Receptor作者:Tzviel Sheskin、Lumir Hanuš、Joram Slager、Zvi Vogel、Raphael MechoulamDOI:10.1021/jm960752x日期:1997.2.1inactive or less active than comparable compounds in the n-6 series. Alkylation or dialkylation of the alpha carbon adjacent to the carbonyl group retains the level of binding in the case of anandamide (compounds 48, 49); however, alpha-monomethylation or alpha,alpha-dimethylation of N-propyl derivatives (50-53) potentiates binding and leads to the most active compounds seen in the present work (Ki values为了建立与脑大麻素受体(CB1)结合的结构要求,我们合成了许多脂肪酸酰胺,乙醇酰胺和一些相关的简单衍生物,并确定了它们的Ki值。还检查了一些α-甲基或α,α-二甲基花生四烯酰基烷基酰胺。在20:4,n-6系列中,未取代的酰胺是惰性的;N-单烷基化,至少直至分支的戊基,导致显着的结合。N,N-二烷基化,在一个烷基上具有或不具有羟基化,导致活性的消除。在ω碳原子上的N-单烷基的羟基化保持活性。在20x,n-6序列中,x必须为3或4;否则,x为3。只有两个双键的存在会导致失活。在n-3系列中 报告的有限数据表明,衍生的乙醇酰胺比n-6系列中的同类化合物无活性或活性较低。对于邻氨基苯甲酰胺(化合物48、49),与羰基相邻的α碳的烷基化或二烷基化保持结合水平。但是,N-丙基衍生物(50-53)的α-单甲基化或α,α-二甲基化可增强结合并导致本研究中活性最高的化合物(Ki值为6.9 +/- 0.7至8
-
Tnf-alpha production inhibitors申请人:——公开号:US20030032623A1公开(公告)日:2003-02-13A purpose of the present invention is to provide TNF-&agr; production inhibitors being useful as therapeutic agents for autoimmune diseases such as rheumatoid arthritis. Novel compounds having the structure represented by the general formula [1] or salts thereof according to the present invention have excellent TNF-&agr; production inhibitory activities, 1 wherein “A” is —(NR 4 )—, —(CR 5 R 6 )— or —O—, “B” is alkylene or alkenylene, R 1 , R 2 , R 4 , R 5 and R 6 are hydrogen, alkyl, alkenyl, adamantyl or the like, R 3 is aryl or an unsaturated heterocycle, and “X” is oxygen or sulfer respectively.
-
[EN] BIARYL COMPOUNDS USEFUL AS IMMUNOMODULATORS<br/>[FR] COMPOSÉS BIARYLES UTILES EN TANT QU'IMMUNOMODULATEURS申请人:BRISTOL MYERS SQUIBB CO公开号:WO2018044963A1公开(公告)日:2018-03-08The present disclosure generally relates to compounds useful as immunomodulators. Provided herein are compounds, compositions comprising such compounds, and methods of their use. The disclosure further pertains to pharmaceutical compositions comprising at least one compound according to the disclosure that are useful for the treatment of various diseases, including cancer and infectious diseases.本公开涉及通常用作免疫调节剂的化合物。本文提供了包含这些化合物的组合物,以及它们的使用方法。该公开进一步涉及包含至少一种根据本公开的化合物的药物组合物,用于治疗各种疾病,包括癌症和传染病。
-
[EN] CHEMICAL COMPOUNDS<br/>[FR] DERIVES DE QUINAZOLINE POUR LE TRAITEMENT DU CANCER申请人:ASTRAZENECA AB公开号:WO2004094410A1公开(公告)日:2004-11-04Quinazoline derivatives of formula (I); for use in the treatment of proliferative diseases such as cancer and in the preparation of medicaments for use in the treatment of proliferative diseases, and to processes for their preparation, as well as pharmaceutical compositions containing them as active ingredient.
表征谱图
-
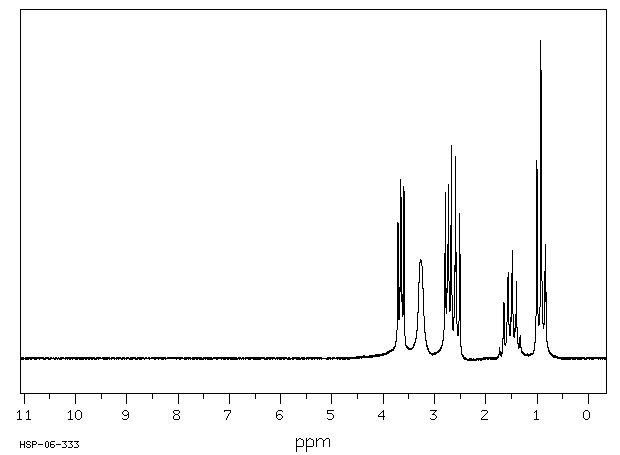
氢谱1HNMR
-
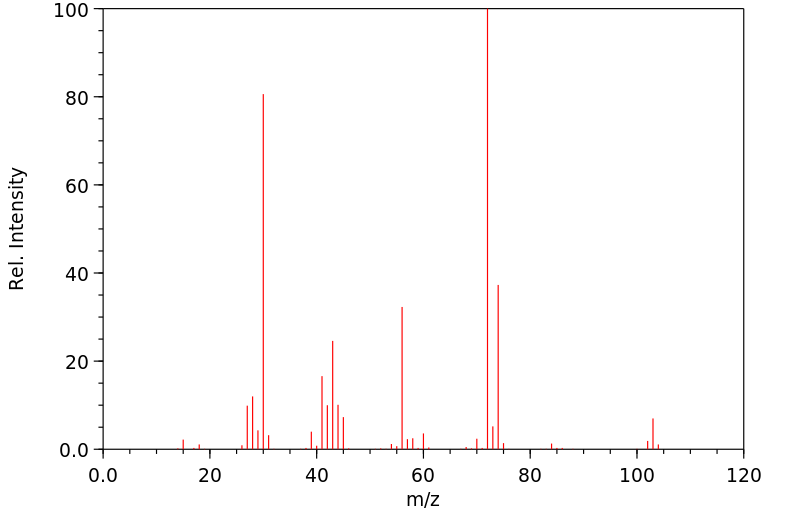
质谱MS
-
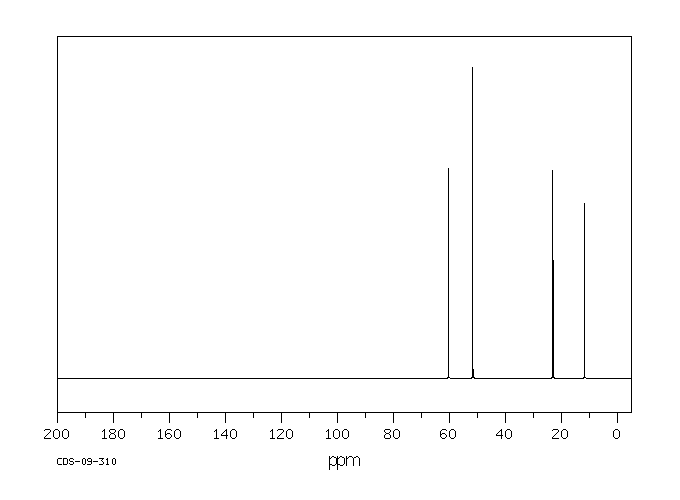
碳谱13CNMR
-
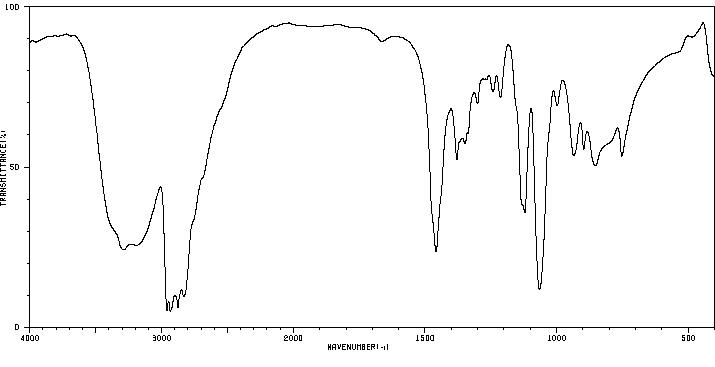
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷