2,2-二甲基庚烷 | 1071-26-7
中文名称
2,2-二甲基庚烷
中文别名
——
英文名称
2,2-dimethylheptane
英文别名
2,2-Dimethyl-heptan
CAS
1071-26-7
化学式
C9H20
mdl
——
分子量
128.258
InChiKey
PSABUFWDVWCFDP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-113 °C
-
沸点:132.7 °C
-
密度:0.7157 g/cm3
-
蒸汽压力:10.77 mmHg
-
保留指数:824.7;818.9;816.53;818.34;820;819;819;817;829.9;813;815;818;820;820;817;820;820;818;815;819.2;816;816;771.4;775.5;814;819;820.3;818;815;817;819;820
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:参考文献:名称:Herington; Rideal, Proceedings of the Royal Society of London, Series A: Mathematical, Physical and Engineering Sciences, 1945, vol. 184, p. 450摘要:DOI:
-
作为产物:参考文献:名称:铜纳米颗粒催化的烷基卤与格利雅试剂的交叉偶联。摘要:在市售铜或氧化铜纳米颗粒作为催化剂和炔烃添加剂的存在下,进行烷基溴和氯化物与各种格氏试剂之间的交叉偶联反应。该催化体系显示出高活性,广泛的范围和良好的官能团耐受性。DOI:10.1039/c3cc46419a
文献信息
-
Alkene oligomerization process申请人:——公开号:US20040006250A1公开(公告)日:2004-01-08A process for oligomerising alkenes having from 3 to 6 carbon atoms which comprises contacting a feedstock comprising a) one or several alkenes having x carbon atoms, and, b) optionally, one or several alkenes having y carbon atoms, x and y being different, with a catalyst containing a zeolite of the MFS structure type, under conditions to obtain selectively oligomeric product containing predominant amounts of certain oligomers. The process is carried out at a temperature comprised between 125 and 175° C. when the feedstock contains only alkenes with 3 carbon atoms and between 140 and 240° C., preferably between 140 and 200° C. when the feedstock contains comprises at least one alkene with 4 or more carbon atoms.一种用于寡聚烯烃的方法,其含有从3到6个碳原子的烯烃,包括将含有a)一个或多个具有x个碳原子的烯烃,以及b)可选地,一个或多个具有y个碳原子的烯烃,其中x和y不同,与含有MFS结构类型沸石的催化剂接触,在条件下,以选择性地获得含有主要某些寡聚体的寡聚产物。当原料只含有3个碳原子的烯烃时,该过程在温度为125至175°C之间进行;当原料含有至少一种含有4个或更多碳原子的烯烃时,该过程在温度为140至240°C之间进行,优选在140至200°C之间进行。
-
TfOH-Catalyzed Cascade Reaction: Metal-Free Access to 3,3-Disubstituted Phthalides from <i>o</i>-Alkynylbenzoic Acids作者:Yanqiu Zhang、Zhiliang Zhang、Yunxin Xia、Jiayi Wang、Yanqing Peng、Gonghua SongDOI:10.1021/acs.joc.3c00760日期:2023.9.153-disubstituted phthalides is reported. A successive reaction process begins with the TfOH-catalyzed cyclization of o-alkynylbenzoic acids followed by an ortho-regioselective electrophilic alkylation of various electron-rich aromatic compounds or alkenes, which has been successfully developed. The corresponding regioselective products of 3-substituted phthalide were obtained in good to high yields.
-
Reetz, Manfred T.; Westermann, Juergen; Steinbach, Rainer, Angewandte Chemie, 1980, vol. 92, # 11, p. 931 - 933作者:Reetz, Manfred T.、Westermann, Juergen、Steinbach, RainerDOI:——日期:——
-
Jamison; Lesslie; Turner, Journal of the Institute of Petroleum, 1949, vol. 35, p. 595作者:Jamison、Lesslie、TurnerDOI:——日期:——
-
Komarewsky; Shand, Journal of the American Chemical Society, 1944, vol. 66, p. 1119作者:Komarewsky、ShandDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
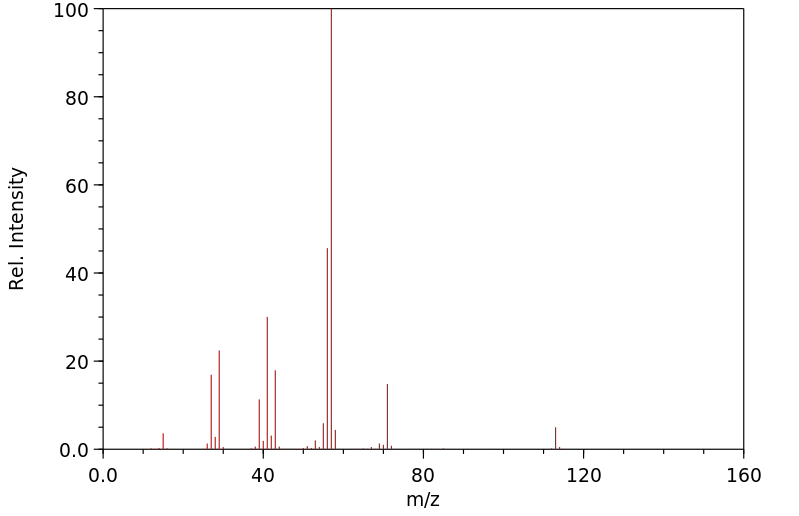
质谱MS
-
碳谱13CNMR
-
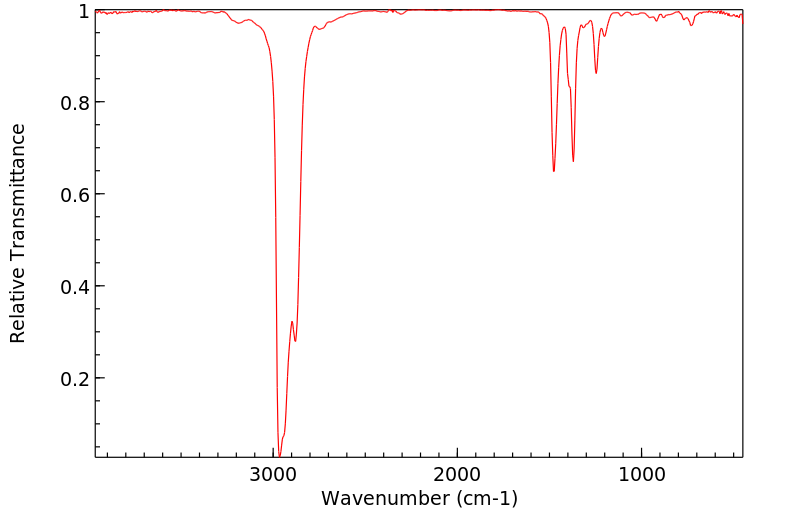
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷