3-甲基-2,5-二氢噻吩-1,1-二氧化物 | 1193-10-8
中文名称
3-甲基-2,5-二氢噻吩-1,1-二氧化物
中文别名
3-甲基-3-环丁烯砜;3-甲基-2,5-二羟基噻吩-1,1-二氧
英文名称
3-methyl-2,5-dihydrothiophen-1,1-dioxide
英文别名
3-methyl-3-sulfolene;3-methylsulfolene;3-methyl-2,5-dihydrothiophene-1,1-dioxide;3-Methyl-2,5-dihydrothiophene 1,1-dioxide
CAS
1193-10-8
化学式
C5H8O2S
mdl
MFCD00005482
分子量
132.183
InChiKey
FAYFWMOSHFCQPG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:62-64°C
-
沸点:214.11°C (rough estimate)
-
密度:1.137 (estimate)
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
物理描述:Solid
-
稳定性/保质期:
如果按照规格使用和储存,则不会发生分解,未有已知危险反应。应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:42.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2934999090
-
储存条件:请将贮藏器密封保存,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-(溴甲基)-2,5-二氢噻吩 1,1-二氧化物 3-(bromomethyl)-2,5-dihydrothiophene 1,1-dioxide 31554-48-0 C5H7BrO2S 211.079 —— 2,3-dimethyl-2,5-dihydrothiophene 1,1-dioxide 10033-87-1 C6H10O2S 146.21
反应信息
-
作为反应物:描述:参考文献:名称:αβ-不饱和环砜的感光光环二聚化。2-环丁砜和噻-2-环己烯-1,1-二氧化物的光二聚体的晶体结构分析。摘要:五元和六元不饱和环状砜在敏化光环二聚反应中的反应性取决于双键的位置和取代。因此,2环丁烯砜(1)和它的六元类似物硫杂-2-环己烯-1,1-二氧化物(3)photodimerize,得到每三个产品,5,6,7和9,10,11,分别其中只有7和10是相似的。但是,3-甲基-2-环丁烯(1a),3-环丁烯(2)及其3-甲基衍生物(2a)以及硫代-3-环己烯-1,1-二氧化物(4)在相同条件下保持不变。1和3的二聚也受到γ辐射的影响。通过晶体结构分析确定了六个二聚体的结构和立体化学。除了6,的γ辐射诱导的二聚化的主要产物1,所有其它的二聚体(5,7和9 - 11)是三环[2 + 2]与在中心环丁烷环的抗(transoid)构cycloadducts。图6是具有CC和CS键收缩的不饱和开链二聚体,后者表明双键与砜基团的共轭。而环丁烷环为5和7(从1开始)是平面的,所有3个从3开始的二聚体都折叠了;6DOI:10.1016/s0040-4020(01)80031-4
-
作为产物:参考文献:名称:750.噻吩的非催化还原。第二部分 2-甲基噻吩,3-甲基噻吩和2:5-二甲基噻吩摘要:DOI:10.1039/jr9510003411
文献信息
-
An Efficient, Overall [4+1] Cycloadditon of 1,3-Dienes and Nitrene Precursors作者:Qiong Wu、Jian Hu、Xinfeng Ren、Jianrong Steve ZhouDOI:10.1002/chem.201101630日期:2011.10.4Intermolecular cycloadditions of conjugated dienes and nitrene precursors usually produce aziridines. A generally useful method was lacking to directly provide the [4+1] cycloadducts, 3‐pyrrolines. We have realized this transformation by using an uniquely active catalyst, copper(II) 1,1,1,5,5,5‐hexafluoroacetylacetonate ([Cu(hfacac)2]). The method is applicable to a wide array of dienes with good yields
-
Efficient synthesis of 3-sulfolenes from allylic alcohols and 1,3-dienes enabled by sodium metabisulfite as a sulfur dioxide equivalent作者:Hang T. Dang、Vu T. Nguyen、Viet D. Nguyen、Hadi D. Arman、Oleg V. LarionovDOI:10.1039/c8ob00745d日期:——We present herein an efficient and practical method for a gram scale synthesis of 3-sulfolenes using sodium metabisulfite as a safe, inexpensive, and easy to handle sulfur dioxide equivalent. Diversely-substituted 3-sulfolenes can be prepared by reacting a variety of 1,3-dienes or allylic alcohols with sodium metabisulfite in aqueous hexafluoroisopropanol (HFIP) or in aqueous methanol in the presence
-
Preparation of α,ω-alkanediylbis-(3-sulfolenes) as precursors for α,ω-bis-(1,3-dienyl)alkanes作者:Ta-shue Chou、Chung-Wen Ko、Teng-Kuei YangDOI:10.1016/s0040-4020(01)81995-5日期:1992.1The sequential substitution reactions of α,ω-diiodoalkanes with two units of 3-sulfolenyl anions conveniently lead to the formation of α,ω-alkanediylbis-(3-sulfolenes) 7. These bissulfolenes are stable precursors for the corresponding bis-(1,3-dienyl)alkanes 16 and the transformation can be readily achieved by a simple thermolytic cheletropic reaction.
-
Small-Ring Compounds. XIV. Radioactive Cyclobutanone from Ketene and Diazomethane-<sup>14</sup>C<sup>1</sup>作者:Dorothy A. Semenow、Eugene F. Cox、John D. RobertsDOI:10.1021/ja01594a069日期:1956.7It has been shown by the ^(14)C-tracer technique that cyclobutanone is formed from the reaction OF ketene with diszomethane-^(14)C via an intermediate possessing the symmetry properties of cyclopropanone.
-
Generation of highly strained 2,3-bridged 2H-azirines via cycloaddition reactions of 2-azidobuta-1,3-dienes and photolysis of the resulting cyclic vinyl azides作者:Klaus Banert、Andreas Ihle、Andrea Kuhtz、Enrico Penk、Biswajit Saha、Ernst-Ulrich WürthweinDOI:10.1016/j.tet.2012.12.054日期:2013.3and with electron-poor dienophiles to generate the Diels–Alder products 12a,b, 13a,b, 14a,b, 15a,b, and 16b. Both types of reactions provide an access to cyclic vinyl azides, which lead to short-lived 2,3-bridged 2H-azirines 18a,b and 20a,b on photolysis. Whereas the highly strained heterocycle 18b could be characterized by low temperature NMR spectra, the bridgehead azirines 18a and 20a,b were trapped用二氧化硫处理2-azidobuta-1,3-dienes 10a,b,制备3-azido-3-sulfolenes 11a,b,并用贫电子的亲二烯体生成Diels–Alder产品12a,b,13a,b,14a,b,15a,b和16b。两种类型的反应都提供了进入环状乙烯基叠氮化物的途径,这导致了短寿命的2,3-桥连2 H-叠氮基18a,b和20a,b光解。高应变的杂环18b可以通过低温NMR光谱表征,而桥头基叠氮基18a和20a,b被环戊二烯通过立体选择性[4 + 2]-环加成法或氰化氢捕获而得到双环2-氰基氮丙啶。后者的转化导致化合物22b显示出温度依赖性的1 H NMR光谱,这是因为氮丙啶N原子上的构型快速平衡。然而,高水平的量子化学研究表明,不仅22b的两种反相异构体(内,外),但每个都涉及两个符合标准的人(椅子,船)。
表征谱图
-
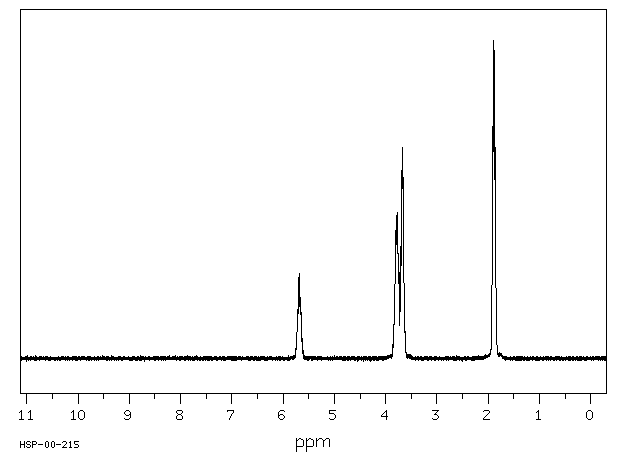
氢谱1HNMR
-
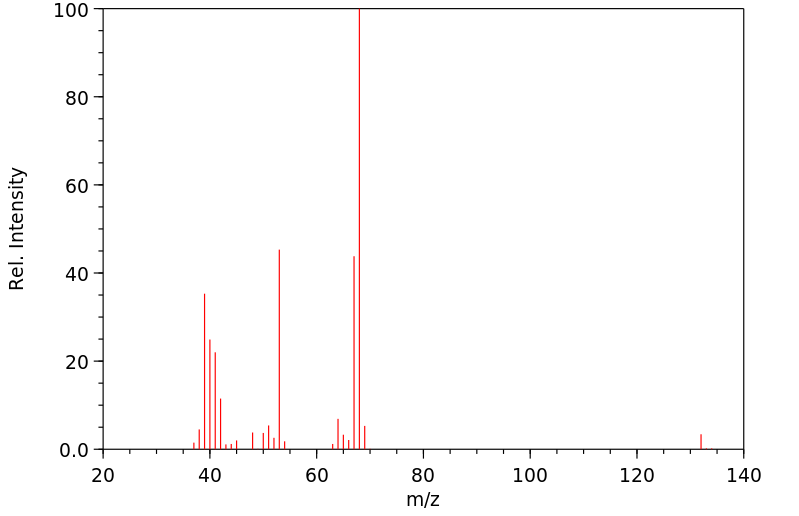
质谱MS
-
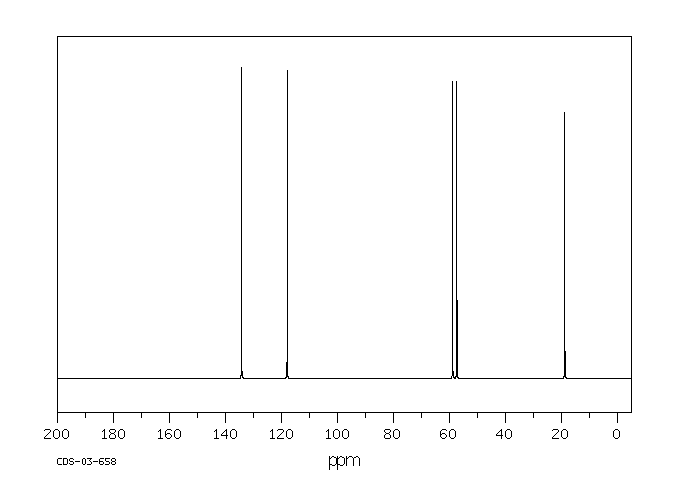
碳谱13CNMR
-
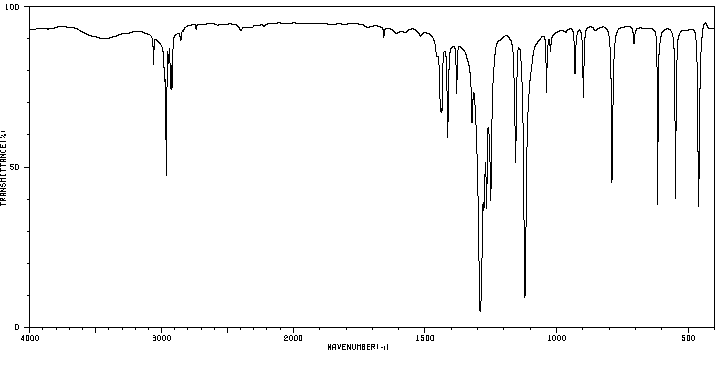
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
硅烷,(2,5-二氢-1,1-二羟基-2-噻嗯基)三甲基-
甲基3-氨基-4,5-二氢-2-噻吩羧酸酯
噻吩,4-(1,1-二甲基乙基)-2,3-二氢-2-甲基-,1,1-二氧化
噻吩,3-氯-4,5-二氢-2-甲基-,1,1-二氧化
噻吩,2,5-二氢-2-甲基-3-(1-甲基乙基)-,1,1-二氧化(9CI)
噻吩,2,5-二氢-2-(2-甲氧基乙基)-3-甲基-,1,1-二氧化
乳霉素
乙基2,5-二氢-3-噻吩羧酸酯
丁酸,2-乙基-2-[(1-羰基丁基)氨基]-
5-羟基-2-甲基-2-辛基噻吩-3-酮
5-甲基-5H-噻吩-2-酮
5-甲基-2,5-二氢噻吩-2-羧酸
5-甲基-2,3-二氢-噻吩
5-甲基-1-氧代-2,3-二氢噻吩-4-羧酸
5-己基-4-甲氧基-5-甲基噻吩-2-酮
5-丁基-3H-噻吩-2-酮
4-甲基-3-氨基二氢噻吩-2-甲酸甲酯
4-甲基-3-叔丁基-5H-噻吩-2-酮
4-甲基-2,5-二氢-噻吩-2-羧酸
4-甲基-2,3-二氢噻吩 1,1-二氧化物
4-(4-氯丁氧基)-5-己基-5-甲基噻吩-2-酮
3-羟基-4-甲基-2(5H)-噻吩酮
3-甲氧基羰基-3-亚砜
3-甲基-2,5-二氢噻吩-1,1-二氧化物
3-甲基-2,5-二氢噻吩
3-环丁烯砜
3-溴-6-甲基-[3,2-B]苯并噻吩-2,5-二酮
3-溴-4-甲基-2,3-二氢噻吩 1,1-二氧化物
3-溴-2,3-二氢-噻吩1,1-二氧化物
3-氯-2,5-二氢-1H-1lambda6-噻吩-1,1-二酮
3-氯-2,3-二氢噻吩 1,1-二氧化物
3-乙基-2,5-二氢噻吩-1,1-二氧化物
3-(溴甲基)-2,5-二氢噻吩 1,1-二氧化物
3-(4-甲基戊-3-烯基)-2,5-二氢噻吩 1,1-二氧化物
3-(2,2-二甲基乙基)-2(5H)-噻吩酮
3-(1,1-二甲基乙基)-3-甲基-2(3H)-噻吩酮
3,4-二氯-2,2,5,5-四氟-2,5-二氢噻吩
3,4-二氟-2,5-噻吩二酮
3,3'-硫代(2,5-二氢噻吩1,1-二氧化物)
3(1H)-异喹啉乙酸,2-[(1,1-二甲基乙氧基)羰基]-3,4-二氢-,(3R)-
2H-环戊二烯并[b]噻吩,2,2,3-三氟-4,5,6,6a-四氢-
2-羟甲基-5-甲基-2,5-二氢噻吩
2-羟甲基-4-甲基-2,5-二氢噻吩
2-甲基-2,5-二氢噻吩
2-溴-4,5-二氢噻吩1,1-二氧化物
2-己基-5-羟基-2-甲基噻吩-3-酮
2-巯基-3,4-二甲基-2,3-二氢噻吩
2,5-二氢噻吩-3-羧酸甲酯
2,5-二氢噻吩-3-甲醛
2,5-二氢噻吩