二苯甲基硅烷 | 776-76-1
中文名称
二苯甲基硅烷
中文别名
二苯基甲基硅烷;甲基二苯基硅烷;二苯基甲基氢硅烷
英文名称
methyldiphenylsilane
英文别名
diphenylmethylsilane;Methyl(diphenyl)silane
CAS
776-76-1
化学式
C13H14Si
mdl
——
分子量
198.34
InChiKey
FULSRCPEOUATID-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:20 °C
-
沸点:93-94 °C1 mm Hg(lit.)
-
密度:0.995 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
在常温常压下保持稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.66
-
重原子数:14
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:1
-
海关编码:29310099
-
危险性防范说明:P305+P351+P338
-
危险性描述:H315,H319
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
| Name: | Diphenylmethylsilane Material Safety Data Sheet |
| Synonym: | Methyldiphenylsilane |
| CAS: | 776-76-1 |
Synonym:Methyldiphenylsilane
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 776-76-1 | Diphenylmethylsilane | >95 | 212-281-9 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 776-76-1: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Negligible.
Specific Gravity/Density: .990 g/ml
Molecular Formula: C13H14Si
Molecular Weight: 198.34
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, silicon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 776-76-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diphenylmethylsilane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 776-76-1: No information available.
Canada
CAS# 776-76-1 is listed on Canada's NDSL List.
CAS# 776-76-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 776-76-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基二苯基氯硅烷 chlorodiphenymethylsilane 144-79-6 C13H13ClSi 232.785 二苯基硅烷 diphenylsilane 775-12-2 C12H12Si 184.313 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二甲基二苯基硅烷 Dimethyldiphenylsilane 778-24-5 C14H16Si 212.367 甲基三苯基硅烷 methyltriphenylsilane 791-29-7 C19H18Si 274.437 —— methyldiphenylsilyl iodide —— C13H13ISi 324.236 甲基二苯基氯硅烷 chlorodiphenymethylsilane 144-79-6 C13H13ClSi 232.785 二苯基甲基硅烷氟 fluoro(methyl)diphenylsilane 17739-53-6 C13H13FSi 216.33 溴-甲基-二苯基-硅烷 Bromo(methyl)diphenylsilane 17571-61-8 C13H13BrSi 277.236 二苯乙烯基甲基硅烷 methyldiphenylvinylsilane 13107-13-6 C15H16Si 224.378 二苯基硅烷 diphenylsilane 775-12-2 C12H12Si 184.313 甲基二苯基硅烷醇 diphenylmethylsilanol 778-25-6 C13H14OSi 214.339 —— Ph2MeSi(18)OH —— C13H14OSi 216.34 —— Methyldiphenylthiosilanol 60642-95-7 C13H14SSi 230.406 —— (4-methoxyphenyl)(methyl)diphenylsilane 14311-79-6 C20H20OSi 304.464 —— allyldiphenyl(methyl)silane 17922-43-9 C16H18Si 238.404 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Co-catalyzed autoxidation of alkene in the presence of silane. The effect of the structure of silanes on the efficiency of the reaction and on the product distribution摘要:A systematic investigation of the structural effect of silanes on the Co-catalyzed reductive oxygenation of alkene in the presence of silane (Mukaiyama-Isayama reaction) showed that the efficiency of the reaction decreases with the increase of the steric bulk of the silanes. A similar trend was observed for the metal-exchange reaction between Co(III)-alkylperoxo complex and silane, too. The peroxidation of (S)-limonene, followed by deprotection of the derived silyl peroxides, provides a mixture of the corresponding monocyclic hydroperoxide 24 and the bicyclic one 25, the ratio being a marked function of the steric bulk of silanes. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2005.08.025
-
作为产物:参考文献:名称:Simplified approach to silaanthrones and disilaanthracenes摘要:DOI:10.1016/0022-328x(84)85185-2
-
作为试剂:参考文献:名称:Intermolecular Antiselective and Enantioselective Reductive Coupling of Enones and Aromatic Aldehydes with Chiral Rh(Phebox) Catalysts摘要:The intermolecular reductive coupling reaction of cyclopent-2-enone and aromatic aldehydes was realized by chiral rhodium-(bisoxazolinyl)phenyl catalysts, Rh(Phebox-Ph)(OAc)(2)(H2O), with diphenymethylsilane as a hydride donor to give the corresponding beta-hydroxyketones in high anti selectivity (up to 96%) with high enantioselectivity (up to 93%).DOI:10.1021/ol802939u
文献信息
-
<i>N</i>-Methyl-Benzothiazolium Salts as Carbon Lewis Acids for Si−H σ-Bond Activation and Catalytic (De)hydrosilylation作者:Valerio Fasano、James E. Radcliffe、Liam D. Curless、Michael J. InglesonDOI:10.1002/chem.201604613日期:2017.1.1N−Me‐Benzothiazolium salts are introduced as a new family of Lewis acids able to activate Si−H σ bonds. These carbon‐centred Lewis acids were demonstrated to have comparable Lewis acidity towards hydride as found for the triarylboranes widely used in Si−H σ‐bond activation. However, they display low Lewis acidity towards hard Lewis bases such as Et3PO and H2O in contrast to triarylboranes. The N−Me‐benzothiazolium
-
Enantioselective Diarylcarbene Insertion into Si–H Bonds Induced by Electronic Properties of the Carbenes作者:Liang-Liang Yang、Declan Evans、Bin Xu、Wen-Tao Li、Mao-Lin Li、Shou-Fei Zhu、K. N. Houk、Qi-Lin ZhouDOI:10.1021/jacs.0c04725日期:2020.7.15enantioselection usually depends on differences in steric interactions between prochiral substrates and a chiral catalyst. We have discovered a carbene Si-H insertion in which the enantioselectivity depends primarily on the electronic characteristics of the carbene substrate, and the log(er) values are linearly related to Hammett parameters. A new class of chiral tetraphosphate dirhodium catalysts was developed
-
Iron-Catalyzed Intermolecular 1,2-Difunctionalization of Styrenes and Conjugated Alkenes with Silanes and Nucleophiles作者:Yuan Yang、Ren-Jie Song、Xuan-Hui Ouyang、Cheng-Yong Wang、Jin-Heng Li、Shenglian LuoDOI:10.1002/anie.201702349日期:2017.6.26The first iron‐catalyzed 1,2‐difunctionalization of styrenes and conjugated alkenes with silanes and either N or C, using an oxidative radical strategy, is described. Employing FeCl2 and di‐tert‐butyl peroxide allows divergent alkene 1,2‐difunctionalizations, including 1,2‐aminosilylation, 1,2‐arylsilylation, and 1,2‐alkylsilylation, which rely on a wide range of nucleophiles, namely, amines, amides
-
Ru(<scp>ii</scp>)-Pheox-catalyzed Si–H insertion reaction: construction of enantioenriched carbon and silicon centers作者:Yoko Nakagawa、Soda Chanthamath、Ikuhide Fujisawa、Kazutaka Shibatomi、Seiji IwasaDOI:10.1039/c7cc01070b日期:——We established a highly enantioselective Si-H insertion reaction to construct chiral centers at the carbon and silicon atoms, using Ru(II)-Pheox catalyst. The catalytic asymmetric Si-H insertion reaction of [small alpha]-methyl-[small alpha]-diazoesters proceeded...我们建立了一个高度对映选择性的Si-H插入反应,使用Ru(II)-Pheox催化剂在碳和硅原子上构建了手性中心。[小α]-甲基-[小α]-重氮酯的催化不对称Si-H插入反应进行...
-
A Free Radical Cascade Silylarylation of Activated Alkenes: Highly Selective Activation of the Si–H/C–H Bonds作者:Lizhi Zhang、Dong Liu、Zhong-Quan LiuDOI:10.1021/acs.orglett.5b01067日期:2015.5.15The first example of silylarylation of activated alkenes with silanes is reported via selective activation of the Si–H/C–H bonds, which allows efficient access to silylated oxindoles through a free-radical cascade process.
表征谱图
-
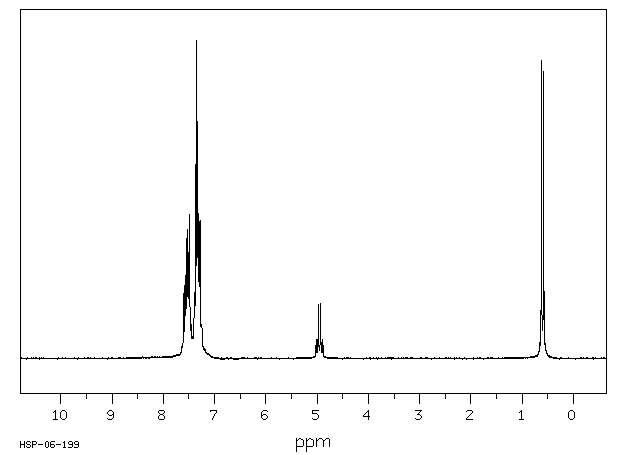
氢谱1HNMR
-
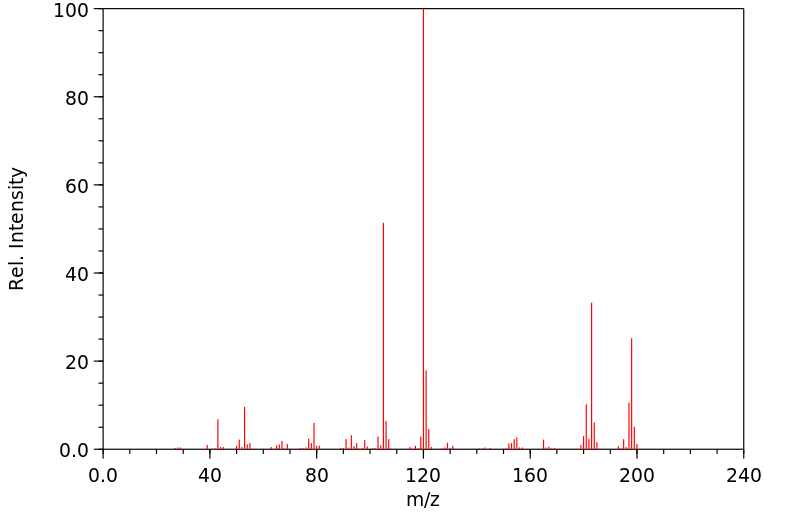
质谱MS
-
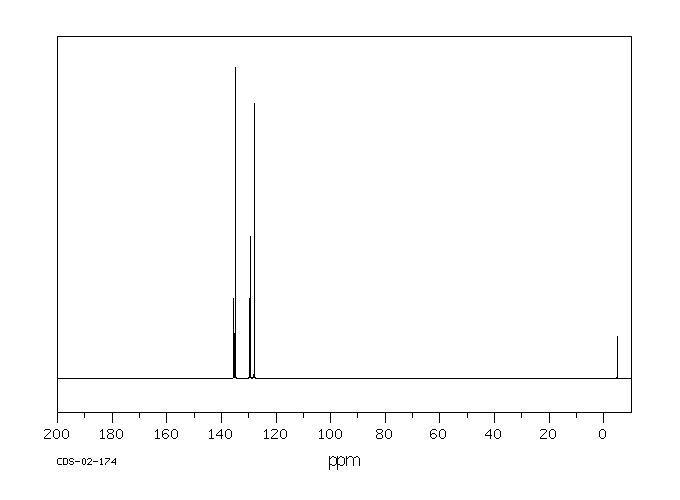
碳谱13CNMR
-
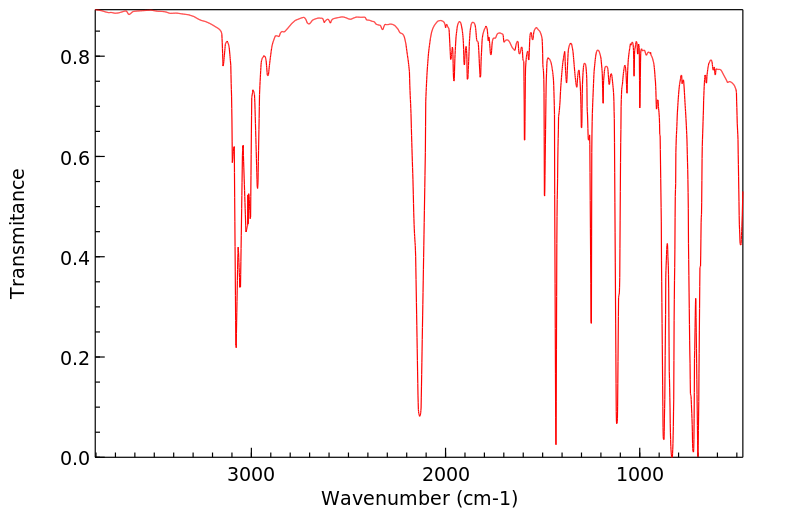
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷