3,4,4-三甲基-1-戊烯-3-醇 | 3732-61-4
中文名称
3,4,4-三甲基-1-戊烯-3-醇
中文别名
——
英文名称
3,4,4-trimethyl-1-penten-3-ol
英文别名
3,4,4-trimethylpent-1-en-3-ol
CAS
3732-61-4
化学式
C8H16O
mdl
——
分子量
128.214
InChiKey
VHCAZBIUJQZQCZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:146-147 °C
-
密度:0.8576 g/cm3(Temp: 11 °C)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905290000
SDS
反应信息
-
作为反应物:描述:参考文献:名称:O 3 / Pb(OAc)4:烯丙醇氧化裂解的新型高效体系摘要:当在温和的条件下用臭氧-乙酸铅(IV)处理时,烯丙醇会发生氧化裂解,从而以高收率获得相应的羰基化合物。DOI:10.1016/j.tetlet.2006.07.020
-
作为产物:描述:3,4,4-三甲基-1-戊炔-3-醇 在 吡啶 、 palladium on activated charcoal 、 Lindlar's catalyst 作用下, 生成 3,4,4-三甲基-1-戊烯-3-醇参考文献:名称:Nasarow et al., Zhurnal Obshchei Khimii, 1955, vol. 25, p. 88,90;engl.Ausg.S.75,77摘要:DOI:
文献信息
-
Rhenium-Catalyzed 1,3-Isomerization of Allylic Alcohols: Scope and Chirality Transfer作者:Christie Morrill、Gregory L. Beutner、Robert H. GrubbsDOI:10.1021/jo061436l日期:2006.9.1The scope of the triphenylsilyl perrhennate (O3ReOSiPh3, 1) catalyzed 1,3-isomerization of allylic alcohols has been thoroughly explored. It was found to be effective for a wide variety of secondary and tertiary allylic alcohol substrates bearing aryl, alkyl, and cyano substituents. Two general reaction types were found which gave high levels of product selectivity: those driven by formation of an
-
Metallorganische verbindungen der lanthanoide作者:Herbert Schumann、Wolfgang Genthe、Ekkehardt Hahn、Joachim Pickardt、Helmut Schwarz、Klaus EckartDOI:10.1016/s0022-328x(00)99709-2日期:1986.5LuCl3 reacts with t-butyllithium in the presence of ether and tetramethylethylene diamine (tmed) to form [Li(tmed)2][Lu(t-C4H9)4], the structure of which has been elucidated through complete X-ray analysis. The crystals are orthorhombic with a 20.995(9), b 18.310(7), c 9.527(2) Å, space group P21ab, Z = 4, D(calcd) 1.17 g cm−3, R = 0.035, and 2471 observed reflections. The compound undergoes 1,2-addition
-
The use of a [4 + 2] cycloaddition reaction for the preparation of a series of ‘tethered’ Ru(<scp>ii</scp>)–diamine and aminoalcohol complexes作者:Fung Kei Cheung、Aidan M. Hayes、David J. Morris、Martin WillsDOI:10.1039/b700744b日期:——A series of catalysts have been prepared for use in the asymmetric transfer hydrogenation of ketones. The complexes were prepared using a [4 + 2] cycloaddition reaction at a key step in the reaction sequence. This provides a means for the synthesis of catalysts with modifications at specific sites.已经制备了一系列用于酮的不对称转移氢化的催化剂。使用[4 + 2]环加成反应在反应顺序的关键步骤制备配合物。这提供了在特定位点进行修饰的合成催化剂的方法。
-
Stereoselective Dehydroxyboration of Allylic Alcohols to Access (<i>E</i>)-Allylboronates by a Combination of C–OH Cleavage and Boron Transfer under Iron Catalysis作者:Wei Su、Ting-Ting Wang、Xia Tian、Jian-Rong Han、Xiao-Li Zhen、Shi-Ming Fan、Ya-Xin You、Yu-Kun Zhang、Rui-Xiao Qiao、Qiushi Cheng、Shouxin LiuDOI:10.1021/acs.orglett.1c03359日期:2021.12.3Iron-catalyzed direct SN2′ dehydroxyboration of allylic alcohols has been developed to access (E)-stereoselective allylboronates. Allylic alcohols with diverse structures and functional groups, especially derived from natural products, underwent smooth transformation. The six-membered ring transition state formed by allylic alcohols and iron–boron intermediate was indicated to be the key component
-
Highly Selective 1,3-Isomerization of Allylic Alcohols via Rhenium Oxo Catalysis作者:Christie Morrill、Robert H. GrubbsDOI:10.1021/ja044054a日期:2005.3.9strategies are developed to promote the highly selective 1,3-isomerization of a variety of allylic alcohols using O3ReOSiPh3 as a catalyst. The first strategy utilizes substrates whose 1,3-regioisomer contains a conjugated alkene, which relies on thermodynamics to obtain high selectivity. The second strategy employs N,O-bis(trimethylsilyl)acetamide as an additive to selectively and irreversibly remove the product
表征谱图
-
氢谱1HNMR
-
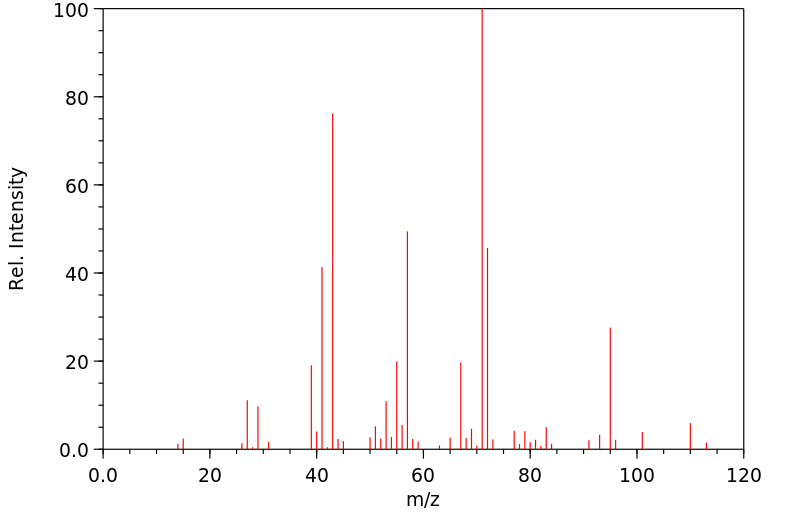
质谱MS
-
碳谱13CNMR
-
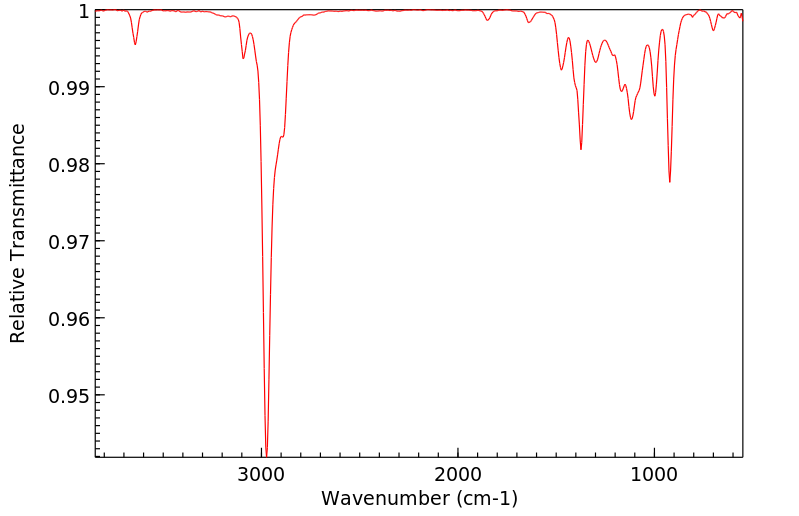
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷