1,8-蒽二羧酸二甲酯 | 93655-34-6
中文名称
1,8-蒽二羧酸二甲酯
中文别名
二甲基1,8-蒽二羧酸酯;1,8-蒽二甲酸二甲酯
英文名称
dimethyl anthracene-1,8-dicarboxylate
英文别名
1,8-dimethylanthracene dicarboxylate;1,8-bis(methoxycarbonyl)anthracene;methyl 1,8-anthracenedicarboxylate;1,8-Dimethoxycarbonyl-anthracen;Dimethyl 1,8-Anthracenedicarboxylate
CAS
93655-34-6
化学式
C18H14O4
mdl
——
分子量
294.307
InChiKey
ALYSIEHCBBJETD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:105 °C
-
沸点:466.7±18.0 °C(Predicted)
-
密度:1.260±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:22
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:52.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
海关编码:2917399090
-
储存条件:室温
SDS
1,8-蒽二羧酸二甲酯
模块 1. 化学品
产品名称: Dimethyl 1,8-ANThracenedicarboxylate
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,8-蒽二羧酸二甲酯
百分比: >95.0%(GC)
CAS编码: 93655-34-6
俗名: 1,8-ANThracenedicarboxylic Acid Dimethyl Ester
分子式: C18H14O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
1,8-蒽二羧酸二甲酯
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-黄色
气味: 无资料
pH: 无数据资料
熔点:
105°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
1,8-蒽二羧酸二甲酯
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1,8-蒽二羧酸二甲酯
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Dimethyl 1,8-ANThracenedicarboxylate
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,8-蒽二羧酸二甲酯
百分比: >95.0%(GC)
CAS编码: 93655-34-6
俗名: 1,8-ANThracenedicarboxylic Acid Dimethyl Ester
分子式: C18H14O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
1,8-蒽二羧酸二甲酯
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-黄色
气味: 无资料
pH: 无数据资料
熔点:
105°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
1,8-蒽二羧酸二甲酯
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1,8-蒽二羧酸二甲酯
模块16 - 其他信息
N/A
制备方法与用途
用途
1,8-蒽二羧酸二甲酯是一种非常有用的有机合成化合物。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,8-蒽二甲酸 anthracene-1,8-dicarboxylic acid 38378-77-7 C16H10O4 266.253 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,8-蒽二甲酸 anthracene-1,8-dicarboxylic acid 38378-77-7 C16H10O4 266.253 1,8-双(羟甲基)蒽 1,8-bis(hydroxymethyl)anthracene 34824-20-9 C16H14O2 238.286 —— 1,8-diformylanthracene 34824-75-4 C16H10O2 234.254
反应信息
-
作为反应物:描述:参考文献:名称:A host–guest system based on collagen-like triple-helix hybridization摘要:基于三螺旋杂交的肽识别策略导致了一个在水介质中表现出高亲和力和选择性的宿主-客体系统。DOI:10.1039/c7cc03055j
-
作为产物:描述:1,8-二羟基蒽醌 在 吡啶 、 sodium 2'-(dicyclohexylphosphanyl)-2,6-diisopropylbiphenyl-4-sulfonate 、 lithium aluminium tetrahydride 、 氯化亚砜 、 水 、 palladium diacetate 、 caesium carbonate 、 sodium hydroxide 、 三氯氧磷 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 甲苯 为溶剂, 反应 24.25h, 生成 1,8-蒽二羧酸二甲酯参考文献:名称:一种识别汞离子的荧光分子、制备方法及应用摘要:本发明公开了一种识别汞离子的荧光分子、制备方法及应用,通过萘环或蒽环等多环芳烃为荧光信号基团作为起始原料,通过一系列优化有机合成反应(取代、加成),连接不同的识别位点后可以得到具有不同识别性能的分子钳状主体化合物;本发明可用于汞离子的检测,解决现有分子器件难以有效识别客体分子的问题。公开号:CN109020878A
文献信息
-
Optically Active Triptycenes, V. Synthesis, Optical Resolution and Absolute Configuration of 2,7-Disubstituted Triptycenes.作者:Masaaki Kuritani、Yoshiteru Sakata、Fumio Ogura、Masazumi NakagawaDOI:10.1246/bcsj.46.605日期:1973.2retaining C2-axis of symmetry was synthesized from 1,5-dichloroanthraquinone. Optical resolution of the triptycene afforded (+)- and (−)-enantiomers. The absolute configuration of (+)-2,7-dicarboxytriptycene was determined as 1R,6R by chemical correlation with (+)-2-methoxy-7-methoxycarbonyltriptycene which has 1R,6S configuration as confirmed by chemical transformation and X-ray structure analysis using
-
[EN] TRIPTYCENE DERIVATIVES FOR NUCLEIC ACID JUNCTION STABILIZATION<br/>[FR] DÉRIVÉS DE TRIPTYCÈNE POUR STABILISER DES JONCTIONS D'ACIDE NUCLÉIQUE申请人:UNIV PENNSYLVANIA公开号:WO2017053982A1公开(公告)日:2017-03-30The present invention is directed to compositions and methods using triptycene derivatives (TCDs) for three way junctions (TWJs).本发明涉及使用三苯基衍生物(TCDs)用于三路交叉点(TWJs)的组合物和方法。
-
Crystalline Hosts Based on the Assembly of Anthracene and Bulky Alcoholic Groups – Host Synthesis, Complex Formation, and X-ray Crystal Structures of Several Inclusion Compounds作者:Edwin Weber、Thomas Hens、Qi Li、Thomas C. W. MakDOI:10.1002/(sici)1099-0690(199905)1999:5<1115::aid-ejoc1115>3.0.co;2-o日期:1999.5A series of new clathrate host molecules (1–10) containing two diarylhydroxymethyl groups attached to different positions (1,5 or 1,8) of a basic anthracene construction unit have been synthesized. Their clathrate formation properties with a variety of organic guests, including amines, alcohols, ketones, and other dipolar aprotic compounds or aromatic hydrocarbons are reported (143 examples of clathrates)
-
(METH)ACRYLATE COMPOUND CONTAINING AROMATIC GROUP申请人:Showa Denko K.K.公开号:EP2397504A1公开(公告)日:2011-12-21Disclosed is a (meth)acrylate compound which can provide a cured article having a high refractive index and excellent transparency and heat resistance, and which has superior handling characteristics; further disclosed are a curable composition containing the (meth)acrylate compound, and a cured article thereof. The (meth) acrylate compound contains an aromatic group represented by the following general formula (1), the curable composition comprising the aromatic-group containing (meth) acrylate compound, and the cured article thereof. (In the formula, R1, R2, R3 and R4 are each independently a hydrogen atom or a methyl group; X is an organic group having an aromatic group and 6 to 30 carbon atoms; and a and b are each independently an integer of 0 to 3.)
-
GAS GENERATING MATERIAL, AND MICROPUMP申请人:Sekisui Chemical Co., Ltd.公开号:EP2860167A1公开(公告)日:2015-04-15Provided is a gas-generating material which can generate a gas in a large amount per unit time and has high storage stability. The gas-generating material 11a according to the present invention comprises a gas-generating agent that is an azo compound or an azide compound, a tertiary amine, a photosensitizing agent and a binder resin.
表征谱图
-
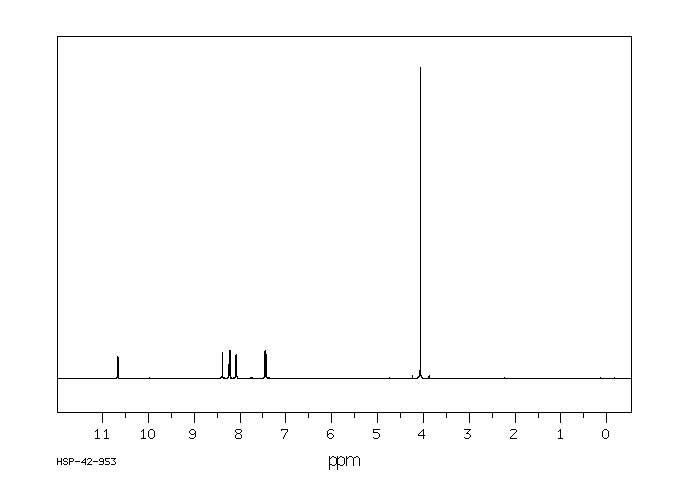
氢谱1HNMR
-
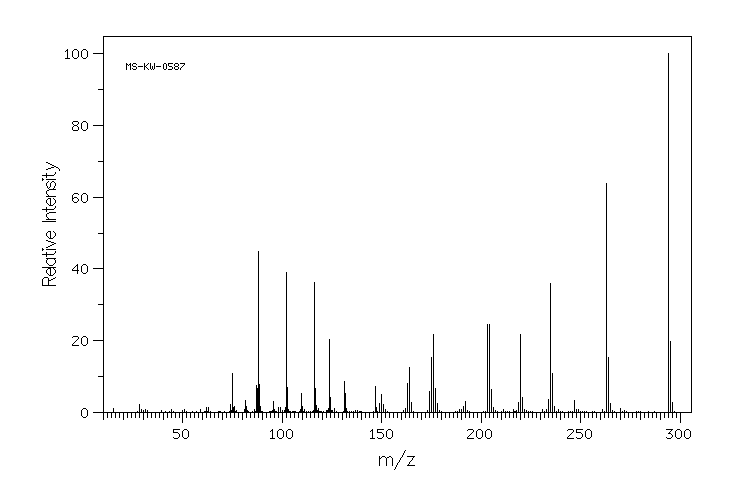
质谱MS
-
碳谱13CNMR
-
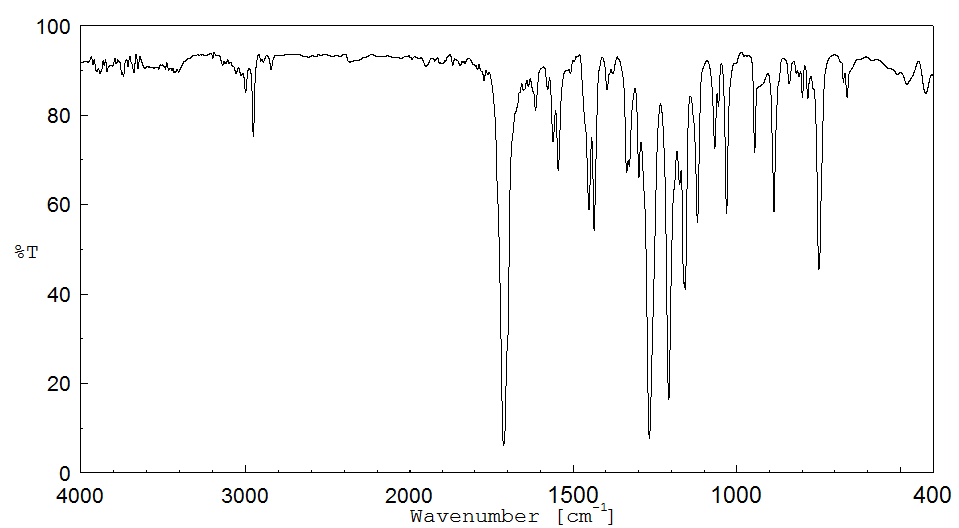
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62