曲安西龙 | 124-94-7
物质功能分类
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:262-263 °C(lit.)
-
比旋光度:69 º (c=2, DMF)
-
沸点:587.5±50.0 °C(Predicted)
-
密度:1.1703 (estimate)
-
溶解度:在DMF中的溶解度为20mg/mL
-
物理描述:Solid
-
颜色/状态:Crystals
-
蒸汽压力:7.20X10-15 mm Hg at 25 °C
-
稳定性/保质期:
Commercially available oral and parenteral preparations of triamcinolone should be stored at a temperature less than 40 °C, preferably between 15-30 °C; freezing of the sterile suspensions should be avoided. Exposure of sterile suspensions of the drug to freezing temperatures can result in irreversible clumping or agglomeration (granular appearance); such suspensions should not be used. Triamcinolone tablets should be stored in well-closed containers. Triamcinolone acetonide sterile suspension should be protected from light. Triamcinolone acetonide oral inhaler should be stored at controlled room temperature (20-25 °C). Because the contents of the oral inhaler are under pressure, the aerosol container should not be punctured, used or stored near heat or an open flame, exposed to temperatures greater than 49 °C, or placed into a fire or incinerator for disposal.
-
旋光度:SPECIFIC OPTICAL ROTATION: +75 DEG @ 25 °C/D (ACETONE); MAX ABSORPTION: 238 NM (E= 15,800)
-
碰撞截面:186.8 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:28
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.71
-
拓扑面积:115
-
氢给体数:4
-
氢受体数:7
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S22,S36
-
危险类别码:R40
-
WGK Germany:3
-
海关编码:2937229000
-
危险品运输编号:OTH
-
RTECS号:TU3850000
SDS
制备方法与用途
醋酸曲安西龙(Triamcinolone)是一种肾上腺皮质激素及促肾上腺皮质激素药物,其抗炎作用比氢化可的松和泼尼松更强,但水钠潴留作用较轻微。口服易吸收,适用于类风湿性关节炎、其他结缔组织疾病、支气管哮喘、过敏性皮炎、神经性皮炎、湿疹等。特别适合对皮质激素禁忌且伴有高血压或水肿的关节炎患者。
适应症利用其强大的免疫抑制作用,醋酸曲安西龙用于治疗各种变态反应性炎症和自身免疫性疾病。其主要药理作用与醋酸泼尼松(强的松)相似,因此适用证也基本相同,主要包括:
- 系统性红斑狼疮等结缔组织病。
- 肾病综合征等免疫性肾脏疾病。
- 特发性血小板减少性紫癜等免疫性疾病。
- 醋酸泼尼松适用的其他疾病。
Triamcinolone是一种糖皮质激素,可采用游离醇或酯化形式口服、肌肉注射、局部注射、吸入或外用。用于治疗多种以皮质类固醇表示的疾病。
| 目标 | 值 |
|---|---|
| 肾上腺素受体 | () |
作为激素类药物使用。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 异氟泼尼松 isoflupredone 338-95-4 C21H27FO5 378.441 6-alpha,9-alpha-二氟-11-beta,16-alpha,17-alpha,21-四羟基孕甾-1,4-二烯-3,20-二酮 6α,9α-difluoro-11β,16α,17α,21-tetra-hydroxypregna-1,4-diene-3,20-dione 807-38-5 C21H26F2O6 412.431 双乙酸呋曲安奈德 triamcinolone diacetate 67-78-7 C25H31FO8 478.515 曲安奈德 triamcinolone acetonide 76-25-5 C24H31FO6 434.505 16alpha-羟基泼尼松龙 desonide 13951-70-7 C21H28O6 376.45 (8S,9S,10R,11S,13S,14S,16R,17S)-11,16,17-三羟基-17-(2-羟基乙酰基)-10,13-二甲基-2,6,7,8,9,11,12,14,15,16-十氢-1H-环戊并[a]菲-3-酮 11β,16α,17α,21-Tetrahydroxy-pregn-4-en-3,20-dion 1171-81-9 C21H30O6 378.466 —— 16α,21-diacetoxyprednisolone 98422-55-0 C25H32O8 460.524 (9beta,11alpha,16alpha)-16,17,21-三羟基-9,11-环氧孕甾a-1,4-二烯-3,20-二酮 9β,11β-epoxy-16α,17α,21-trihydroxy-pregna-1,4-diene-3,20-dione 94088-91-2 C21H26O6 374.434 (16alpha)-16,17-二羟基-3,20-二氧代孕甾烷-4,9(11)-二烯-21-基乙酸酯 21-Acetoxy-16α,17α-dihydroxy-4,9(11)-pregnadien-3,20-dion 74220-43-2 C23H30O6 402.488 —— 9α-bromoprednisolone-16,21-diacetate 91160-86-0 C25H31BrO8 539.42 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Pentanoic acid 2-((8S,9R,10S,11S,13S,14S,16R,17S)-9-fluoro-11,16,17-trihydroxy-10,13-dimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxo-ethyl ester 10452-96-7 C26H35FO7 478.558 (8S,9R,10S,11S,13S,14S,16R,17S)-9-氟-11,16,17-三羟基-17-(2-羟基乙酰基)-10,13-二甲基-1,2,6,7,8,11,12,14,15,16-十氢环戊烯并[a]菲-3-酮 9α-fluoro-11β,16α,17α,21-tetrahydroxypregn-4-ene-3,20-dione 337-02-0 C21H29FO6 396.456 曲安奈德 triamcinolone acetonide 76-25-5 C24H31FO6 434.505 安西法尔 16α,17-(1-ethyl-propylidenedioxy)-9-fluoro-11β,21-dihydroxy-pregna-1,4-diene-3,20-dione 3924-70-7 C26H35FO6 462.559 醋酸曲安奈德 triamcinolone acetonide acetate 3870-07-3 C26H33FO7 476.542 —— (6aS,6bR,7S,8aS,8bS,10R,11aR,12aS,12bS)-10-(4-(3-aminobenzyl)phenyl)-6b-fluoro-7-hydroxy-8b-(2-hydroxyacetyl)-6a,8a-dimethyl-1,2,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-4H-naphtho[2′,1′:4,5]indeno[1,2-d][1,3]dioxol-4-one —— C35H38FNO6 587.688 —— (6aS,6bR,7S,8aS,8bS,10R,11aR,12aS,12bS)-10-(4-((3-aminophenyl)thio)phenyl)-6b-fluoro-7-hydroxy-8b-(2-hydroxyacetyl)-6a,8a-dimethyl-1,2,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-4H-naphtho[2′,1′:4,5]indeno[1,2-d][1,3]dioxol-4-one —— C34H36FNO6S 605.727 —— 9-fluoro-11β,21-dihydroxy-16α,17-((Ξ)-1-phenyl-ethylLiDenedioxy)-pregna-1,4-diene-3,20-dione 3092-82-8 C29H33FO6 496.576 氟氢缩松 flurandrenolide 1524-88-5 C24H33FO6 436.521
反应信息
-
作为反应物:描述:曲安西龙 在 双氧水 、 potassium carbonate 作用下, 以 甲醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 23.0h, 以45%的产率得到9α-fluoro-16α,17α,11β-trihydroxy-3-oxoandrosta-1,4-diene-17β-carboxylic acid参考文献:名称:抗炎的17β-硫代烷基-16α,17α-缩酮和-缩醛雄酮类:一类新的用于治疗哮喘的气道选择性类固醇。摘要:描述了新型的17β-硫代烷基-16α,17α-缩酮和-缩醛雄烷烃的合成和抗炎作用。这类新的类固醇是通过将相应的17个酸的2-硫代-1,2-二氢吡啶-1-基酯片段化为17-自由基制成的。使用多种亲二硫键捕获生成的自由基,得到在C-16和C-17处具有乙缩醛或缩酮功能的甾族D环,以及在C-17处具有硫键。该系列化合物与糖皮质激素受体高效结合,是功能性激动剂,通过在体外诱导大鼠肝细胞系中酪氨酸氨基转移酶活性的能力来衡量。当直接施用于呼吸道时,这些17β-硫代烷基雄烷酮可有效抑制Sephadex诱导的大鼠肺部炎症。与目前可获得的吸入式糖皮质激素相比,高的局部效力以及诱导全身性类糖皮质激素样副作用(大鼠胸腺退化)的低倾向使本发明化合物具有高度的气道选择性。本文所述的17β-硫代烷基-16α,17α-缩酮雄烷酮可用于治疗炎性疾病例如哮喘。DOI:10.1021/jm9604639
-
作为产物:参考文献:名称:ALKALINE REARRANGEMENT OF PHENYL GROUPS LINKED TO SILICON摘要:DOI:10.1021/ja01514a062
文献信息
-
DISUBSTITUTED TRIFLUOROMETHYL PYRIMIDINONES AND THEIR USE申请人:BAYER PHARMA AKTIENGESELLSCHAFT公开号:US20160221965A1公开(公告)日:2016-08-04The present application relates to novel 2,5-disubstituted 6-(trifluoromethyl)pyrimidin-4(3H)-one derivatives, to processes for their preparation, to their use alone or in combinations for the treatment and/or prevention of diseases, and to their use for preparing medicaments for the treatment and/or prevention of diseases, in particular for treatment and/or prevention of cardiovascular, renal, inflammatory and fibrotic diseases.
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOCAD JOINT STOCK CO公开号:WO2018092047A1公开(公告)日:2018-05-24The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.本发明涉及一种新的化合物,其化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为CH2,NH,O或化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[化学式II]每个R7,R8,R9,R10独立地为乙烯基,甲基乙炔基;Hal为CI,Br,I,F,具有布鲁顿酪氨酸激酶(Btk)抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。
-
SULFOXIMINE SUBSTITUTED QUINAZOLINES FOR PHARMACEUTICAL COMPOSITIONS申请人:BLUM Andreas公开号:US20140135309A1公开(公告)日:2014-05-15This invention relates to novel sulfoximine substituted quinazoline derivatives of formula I wherein Ar, R 1 and R 2 are as defined herein, and their use as MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
[EN] SULFOXIMINE SUBSTITUTED QUINAZOLINES FOR PHARMACEUTICAL COMPOSITIONS<br/>[FR] QUINAZOLINES SUBSTITUÉES PAR SULFOXIMINE POUR COMPOSITIONS PHARMACEUTIQUES申请人:BOEHRINGER INGELHEIM INT公开号:WO2014072244A1公开(公告)日:2014-05-15This invention relates to novel sulfoximine substituted quinazoline derivatives of formula (I), wherein Ar, R1 and R2 are as defined in the description and claims, and their use as MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) kinase inhibitors, pharmaceutical compositions containing the same, and methods of using the same as agents for treatment or amelioration of MNK1 (MNK1a or MNK1b) and/or MNK2 (MNK2a or MNK2b) mediated disorders.
-
[EN] ARYL ETHER-BASE KINASE INHIBITORS<br/>[FR] INHIBITEURS DE KINASES DE TYPE ARYLÉTHER-BASE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2015038112A1公开(公告)日:2015-03-19The present disclosure is generally directed to compounds which can inhibit AAK1 (adaptor associated kinase 1), compositions comprising such compounds, and methods for inhibiting AAK1.
表征谱图
-
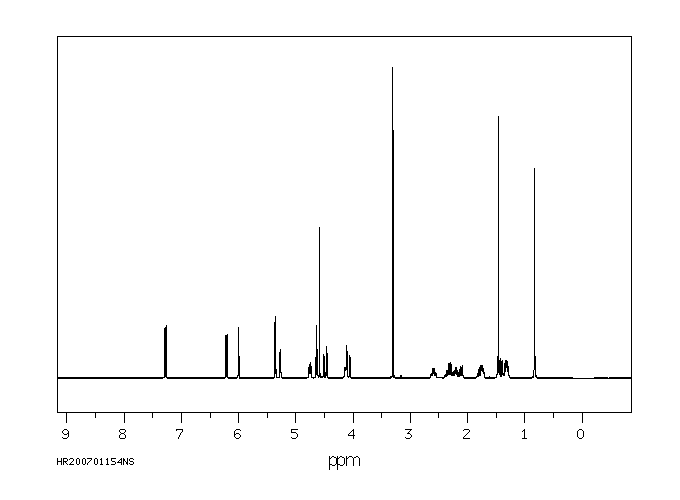
氢谱1HNMR
-
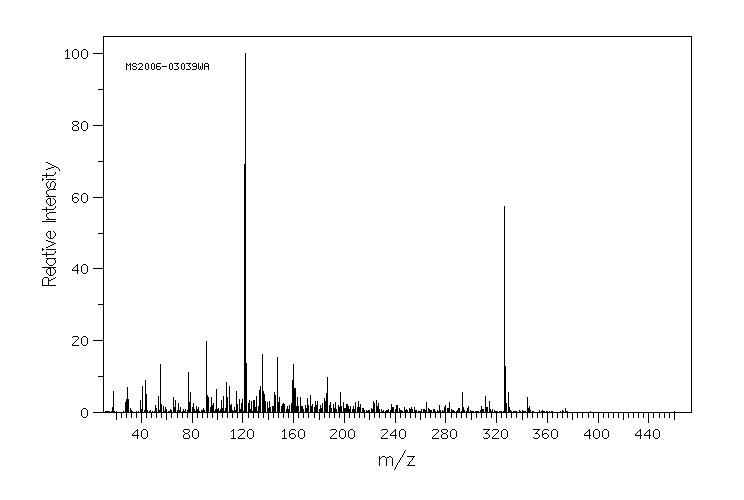
质谱MS
-
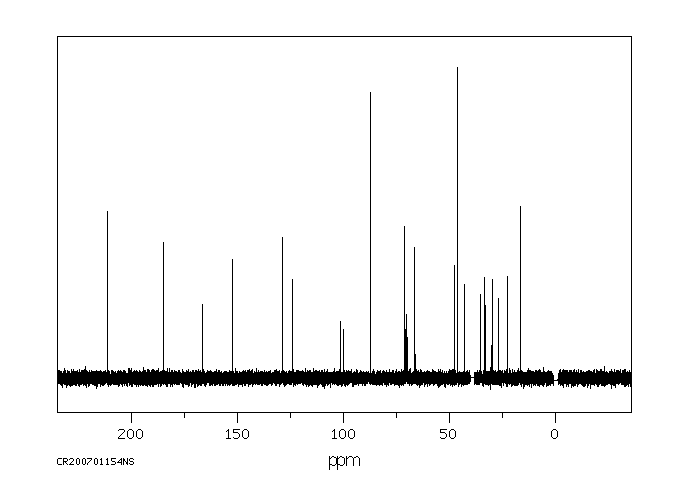
碳谱13CNMR
-
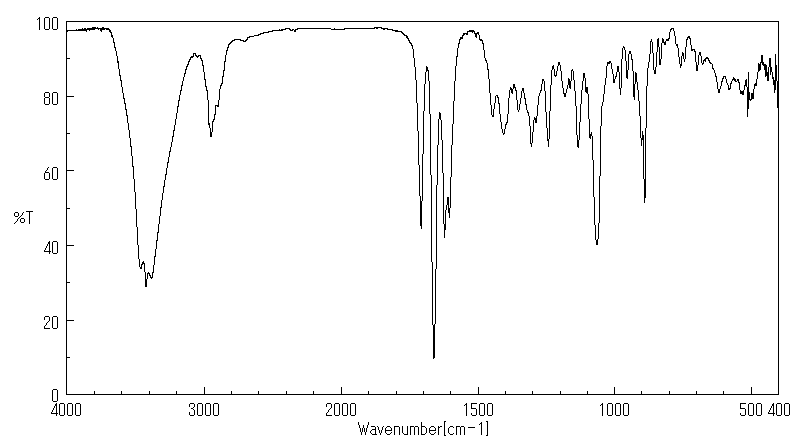
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息