1-(2-溴乙氧基)-4-甲氧基苯 | 22921-76-2
中文名称
1-(2-溴乙氧基)-4-甲氧基苯
中文别名
——
英文名称
1-(2-bromoethoxy)-4-methoxybenzene
英文别名
2-(4-methoxyphenoxy)ethyl bromide;2-(4-methoxyphenyloxy)ethyl bromide
CAS
22921-76-2
化学式
C9H11BrO2
mdl
MFCD00017893
分子量
231.089
InChiKey
PRRJGZRMBVOTGG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:46-50 °C
-
沸点:140-142 °C (10 mmHg)
-
密度:1.382±0.06 g/cm3(Predicted)
-
闪点:150 °C
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2909309090
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:存放于阴凉干燥处即可。
SDS
| Name: | 1-(2-Bromoethoxy)-4-methoxybenzene Material Safety Data Sheet |
| Synonym: | |
| CAS: | 22921-76-2 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 22921-76-2 | 1-(2-Bromoethoxy)-4-methoxybenzene | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 22921-76-2: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 140 - 142 deg C @10mmHg
Freezing/Melting Point: 48 - 49 deg C
Autoignition Temperature: Not available.
Flash Point: 150 deg C ( 302.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: >250 deg C
Solubility in water:
Specific Gravity/Density:
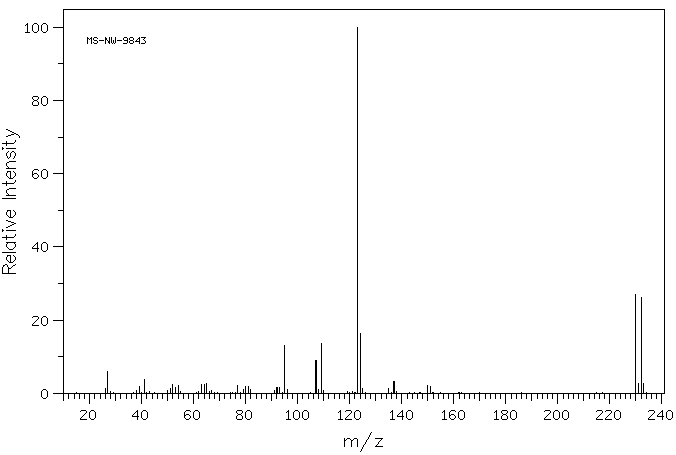
Molecular Formula: C9H11BrO2
Molecular Weight: 231.09
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 22921-76-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(2-Bromoethoxy)-4-methoxybenzene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 22921-76-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 22921-76-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 22921-76-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(4-甲氧基苯氧基)-1-乙醇 2-(4-methoxyphenoxy)ethanol 5394-57-0 C9H12O3 168.192 4-甲氧基苯酚 4-methoxy-phenol 150-76-5 C7H8O2 124.139 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(4-甲氧基苯氧基)乙胺 2-(4-methoxyphenoxy)ethylamine 50800-92-5 C9H13NO2 167.208 3-(4-甲氧基苯氧基)丙腈 3-(4-methoxyphenoxy)propanenitrile 63815-39-4 C10H11NO2 177.203 2-(4-甲氧基苯氧基)-n-甲基-1-乙胺 2-(4-methoxyphenoxy)-N-methylethan-1-amine 102246-82-2 C10H15NO2 181.235 —— 2-(4-Methoxy-phenoxy)-ethylhydrazin 91054-79-4 C9H14N2O2 182.222 —— 2-[2-(4-methoxy-phenoxy)-ethylamino]-ethanol 101568-63-2 C11H17NO3 211.261 2-(4-甲氧基苯氧基)-N,N-二甲基-乙胺 2-(4-methoxyphenoxy)-N,N-dimethylethanamine 51344-12-8 C11H17NO2 195.261 N,N-二乙基-2-(4-甲氧基苯氧基)乙胺 N,N-diethyl-2-(4'-methoxyphenoxy)aminoethane 2759-98-0 C13H21NO2 223.315 2-{乙基-[2-(4-甲氧基-苯氧基)-乙基]-氨基}-乙醇 2-{ethyl-[2-(4-methoxy-phenoxy)-ethyl]-amino}-ethanol 102237-12-7 C13H21NO3 239.315 —— 1-methoxy-4-(vinyloxy)benzene 4024-19-5 C9H10O2 150.177
反应信息
-
作为反应物:描述:1-(2-溴乙氧基)-4-甲氧基苯 在 氢溴酸 、 三乙胺 作用下, 以 乙醇 、 二氯甲烷 、 水 为溶剂, 反应 56.0h, 生成 thiophen-2-yl(4-(2-(4-methoxyphenoxy)ethyl)piperazin-1-yl)methanone参考文献:名称:发现1-芳氧基乙基哌嗪衍生物作为Kv1.5钾通道抑制剂(第一部分)摘要:Kv1.5钾通道是一种治疗房颤(AF)的有效且安全的治疗靶标,它是威胁人类的最常见心律不齐。本文中,通过从内部数据库中修改命中化合物7k,合成了48种衍生物,以通过全细胞膜片钳技术测定其Kv1.5抑制作用。选择了六种显示出比阳性化合物决奈达隆更好的效价的化合物用于其类药物性质的下一个评估。化合物8显示出平衡的溶解度和渗透性。它还显示出可接受的药效学特征,急性毒性非常低。考虑到所有这些数据,化合物8 可以作为开发用于治疗AF的新型治疗剂的有希望的先导。DOI:10.1016/j.ejmech.2014.03.075
-
作为产物:描述:参考文献:名称:基于1,4-二甲氧基苯氧化还原基团的电活性咪唑鎓盐:合成与电化学表征†摘要:已经基于1,4-二甲氧基苯(1)和2,5-二叔丁基-1,4-二甲氧基苯(2和3)合成了三种新的氧化还原活性离子盐。这些咪唑鎓盐是要结合到离子液体结构中的第一个有机氧化还原活性基团。通过将2,5-二叔丁基-1,4-二甲氧基苯引入电活性咪唑鎓盐中,对传输性能没有负面影响。热稳定性和氧化电势已提高。DOI:10.1039/c3ra41345d
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] CARBIDOPA PRODRUGS AND USES THEREOF<br/>[FR] PROMEDICAMEMTS DE CARBIDOPA ET LEURS UTILISATIONS申请人:XENOPORT INC公开号:WO2004052841A1公开(公告)日:2004-06-24Prodrugs of carbidopa, derivatives of carbidopa prodrugs, methods of making prodrugs of carbidopa and derivatives thereof, methods of using prodrugs of carbidopa and derivatives thereof, and compositions of prodrugs of carbidopa and derivatives thereof are disclosed.
-
Synthesis, Biological Activity Evaluation and Molecular Modeling Study on the New Isoconessimine Derivatives as Acetylcholinesterase Inhibitors作者:Guofei Jin、Zhongduo Yang、Weiwei Xue、Jie Sheng、Yin Shi、Xiaojun YaoDOI:10.1002/cjoc.201300441日期:2013.9110 nmol/L which is close to that of reference compound huperzine A (IC50=70 nmol/L). The mode of AChE inhibition by 7b was reversible and non‐competitive. In addition, molecular modeling was performed to explore the binding mode of inhibitor 7b at the active site of AChE and the results showed that 7b could be docked into the acetylcholinesterase active site and compound 7b had hydrophobic interactions新的异cosnessimine衍生物是从conessine(1)合成的,并被评估为乙酰胆碱酯酶(AChE)抑制剂。衍生物是通过两个反应步骤(N-去甲基化和亲核取代)制备的。所有合成的衍生物均显示出比Conessine(1)(IC 50 = 16 µmol·L -1)和异椰油亚胺(2)(IC 50 > 300 µmol·L -1)更高的潜在抗乙酰胆碱酯酶活性。化合物7b(3β- [甲基-[2- [4-(4-硝基苯氧基)乙基]氨基] con-5-烯酸)对IC 50的抑制作用最强110 nmol / L的浓度接近参考化合物石杉碱A的浓度(IC 50 = 70 nmol / L)。7b抑制AChE的模式是可逆的,并且是非竞争性的。此外,进行分子建模以探索抑制剂7b在AChE活性位点的结合方式,结果表明7b可以与乙酰胆碱酯酶活性位点对接,化合物7b与Trp279和Leu282发生疏水相互作用。
-
Novel cyclic amide derivatives申请人:——公开号:US20030212094A1公开(公告)日:2003-11-13Novel compounds represented by the following formula (I) that act as a ligand to sigma receptor/binding cite and a medicament comprising the same as an active ingredient: 1 wherein X represents an alkyl group, an aryl group, a heterocyclic group or the like; Q represents a group represented by —CH 2 —, —CO—, —O—, —CH(OR 7 )— or the like wherein R 7 represents a hydrogen atom, an alkyl group or the like; n represents an integer of from 0 to 5; R 1 and R 2 each represent a hydrogen atom, an alkyl group or the like; B represents either of the following groups: 2 wherein R 3 , R 4 , R 5 , and R 6 each represent a hydrogen atom, a halogen atom, an alkoxyl group or the like; m represents 1 or 2; and the ring of: 3 represents an aromatic heterocyclic ring.以下公式(I)表示的新化合物作为sigma受体/结合位点的配体,并包括作为活性成分的药物: 其中X代表烷基、芳基、杂环基或类似基团;Q代表由—CH 2 —、—CO—、—O—、—CH(OR 7 )—或类似基团表示的基团,其中R 7 代表氢原子、烷基或类似基团;n代表从0到5的整数;R 1 和R 2 各自代表氢原子、烷基或类似基团;B代表以下任一基团: 其中R 3 、R 4 、R 5 和R 6 各自代表氢原子、卤素原子、烷氧基或类似基团;m代表1或2;以及: 代表芳香杂环环。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
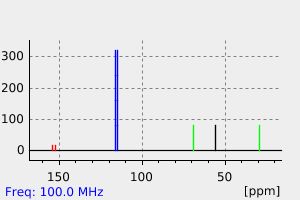
碳谱13CNMR
-
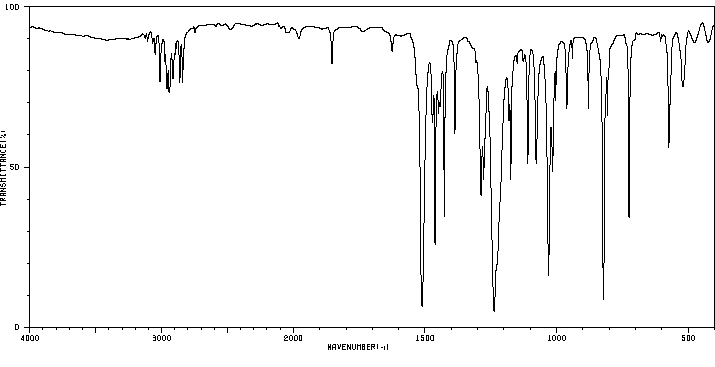
红外IR
-
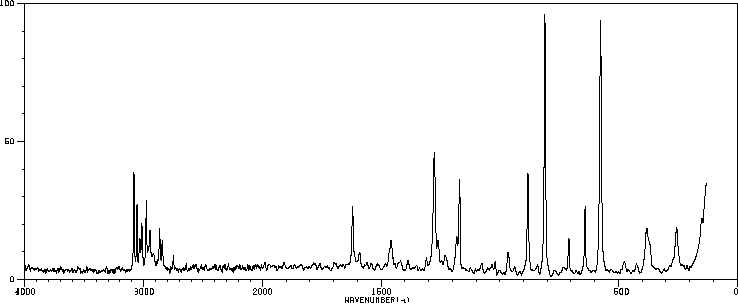
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯