5-庚基苯-1,3-二醇 | 500-67-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:55-57°C
-
沸点:342.3±12.0 °C(Predicted)
-
密度:1.033±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:15
-
可旋转键数:6
-
环数:1.0
-
sp3杂化的碳原子比例:0.54
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2907299090
SDS
制备方法与用途
5-庚基苯-1,3-二醇可用作有机合成与医药化学中间体。它可用于合成各种类型的有机分子,例如聚合物、涂料、树脂和生物活性分子等。
合成方法在干燥的反应烧瓶中,将三溴化硼(11 mL,11 mmol,1 M CH2Cl2溶液)加入到3,5-二甲氧基-1-正庚基苯(5 mmol)的干燥二氯甲烷(40 mL)溶液中。随后,在氩气气氛下将反应混合物置于−78℃反应。逐步提高反应温度至0℃,并在此温度下继续搅拌直至反应完成(大约28小时)。接着,将反应体系转移至-78°C冷井,并加入甲醇以破坏未反应的三溴化硼。恢复至室温后,搅拌40分钟。随后,在真空条件下除去挥发物。用乙酸乙酯稀释残余物,依次用饱和NaHCO3溶液、水和盐水洗涤。分离有机层并使用无水Na2SO4进行干燥,过滤去除干燥剂。最后,将所得滤液在真空下减压浓缩,并通过硅胶柱色谱(45%乙醚/石油醚)进行纯化,即可得到目标产物分子5-庚基苯-1,3-二醇。
上下游信息
反应信息
-
作为反应物:描述:5-庚基苯-1,3-二醇 在 palladium on activated charcoal 4-二甲氨基吡啶 、 氢气 、 对甲苯磺酸 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 作用下, 以 甲醇 、 乙醇 、 二氯甲烷 为溶剂, 25.0 ℃ 、101.33 kPa 条件下, 反应 42.5h, 生成 KS-501参考文献:名称:Total syntheses of KS-501, KS-502, and their enantiomers摘要:The total syntheses of the title compounds are described. The key step involves the coupling of the anhydrofuranose 18 (as well as its enantiomer) with salicylate derivative 17 under the influence of potassium carbonate. Both the epoxidation of 7 (achieved with 2,2-dimethyldioxirane) and the glycosylation of 17 appear to be stereospecific.DOI:10.1021/ja00028a035
-
作为产物:参考文献:名称:Synthesis of 3,5,7-triketo acids and esters and their cyclizations to resorcinol and phloroglucinol derivatives. Models of biosynthesis of phenolic compounds摘要:DOI:10.1021/ja01001a060
文献信息
-
Synthesis and pharmacology of the isomeric methylheptyl-Δ8-tetrahydrocannabinols作者:John W. Huffman、John Liddle、Sammy G. Duncan、Shu Yu、Billy R. Martin、Jenny L. WileyDOI:10.1016/s0968-0896(98)80014-x日期:1998.12The synthesis of the 3-heptyl, and the eleven isomeric 3-methylheptyl-delta8-tetrahydrocannabinols (3-7, R and S methyl epimers, and 8) has been carried out. The synthetic approach entailed the synthesis of substituted resorcinols, which were subjected to acid catalyzed condensation with trans-para-menthadienol to provide the delta8-THC analogue. The 1'-, 2'- and 3'-methylheptyl analogues (3-5) are
-
Using (+)-carvone to access novel derivatives of (+)-ent-cannabidiol: The first asymmetric syntheses of (+)-ent-CBDP and (+)-ent-CBDV作者:Alexandra E. Golliher、Antonio J. Tenorio、Nina O. Dimauro、Nicolas R. Mairata、F. Omar Holguin、William MaioDOI:10.1016/j.tetlet.2021.152891日期:2021.3has a higher affinity to CB1/CB2 receptors than the natural stereoisomer. We have developed an inexpensive, stereoselective route to access ent-CBD derivatives using (+)-carvone as a starting material. In addition to (+)-CBD, we report the first syntheses of (+)-cannabidivarin, (+)-cannabidiphorol as well as C-6/C-8 homologues.
-
MODIFIER FOR AROMATIC POLYESTER AND AROMATIC POLYESTER RESIN COMPOSITION COMPRISING THE SAME申请人:TABATA Masayoshi公开号:US20110224343A1公开(公告)日:2011-09-15The present invention provides a modifier for aromatic polyesters which enhances the melt fluidity of aromatic polyesters without a significant decrease in the heat resistance of the aromatic polyesters, and an aromatic polyester resin composition including the modifier for aromatic polyesters. The present invention relates to a modifier for aromatic polyesters comprising polyhydric phenol residues and residues of aromatic polycarboxylic acid, acid halide or acid anhydride thereof, and the modifier comprises a material having a structure composed of a first residue selected from the group consisting of divalent residues represented by Formula (I): —Ar—W 1 x —Ar— and by Formula (II): —Ar—, the first residues being bonded to two identical or different second residues selected from the group consisting of monovalent residues represented by Formula (III): and monovalent residues represented by Formula (IV): —O—C(O)—R 7 —.
-
大麻二酚衍生物及其制备方法和在医药上的应用
-
Oxidative Stress and Multi-Organel Damage Induced by Two Novel Phytocannabinoids, CBDB and CBDP, in Breast Cancer Cells作者:Maria Salbini、Alessandra Quarta、Fabiana Russo、Anna Maria Giudetti、Cinzia Citti、Giuseppe Cannazza、Giuseppe Gigli、Daniele Vergara、Antonio GaballoDOI:10.3390/molecules26185576日期:——
Over the last few years, much attention has been paid to phytocannabinoids derived from Cannabis for their therapeutic potential. Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the most abundant compounds of the Cannabis sativa L. plant. Recently, novel phytocannabinoids, such as cannabidibutol (CBDB) and cannabidiphorol (CBDP), have been discovered. These new molecules exhibit the same terpenophenolic core of CBD and differ only for the length of the alkyl side chain. Roles of CBD homologs in physiological and pathological processes are emerging but the exact molecular mechanisms remain to be fully elucidated. Here, we investigated the biological effects of the newly discovered CBDB or CBDP, compared to the well-known natural and synthetic CBD (nat CBD and syn CBD) in human breast carcinoma cells that express CB receptors. In detail, our data demonstrated that the treatment of cells with the novel phytocannabinoids affects cell viability, increases the production of reactive oxygen species (ROS) and activates cellular pathways related to ROS signaling, as already demonstrated for natural CBD. Moreover, we observed that the biological activity is significantly increased upon combining CBD homologs with drugs that inhibit the activity of enzymes involved in the metabolism of endocannabinoids, such as the monoacylglycerol lipase (MAGL) inhibitor, or with drugs that induces the activation of cellular stress pathways, such as the phorbol ester 12-myristate 13-acetate (PMA).
在过去几年里,人们对来自大麻的植物类大麻素的治疗潜力给予了很多关注。 Δ9-四氢大麻酚(Δ9-THC)和大麻二酚(CBD)是大麻植物中最丰富的化合物。最近,发现了新型植物类大麻素,如大麻二丁醇(CBDB)和大麻二酚(CBDP)。这些新分子展示了与CBD相同的萜烯酚核心,只是烷基侧链长度不同。CBD同系物在生理和病理过程中的作用正在浮出水面,但确切的分子机制尚待完全阐明。在这里,我们研究了新发现的CBDB或CBDP的生物效应,与已知的天然和合成CBD(nat CBD和syn CBD)在表达CB受体的人类乳腺癌细胞中进行了比较。具体来说,我们的数据表明,用新型植物类大麻素处理细胞会影响细胞的存活能力,增加活性氧(ROS)的产生,并激活与ROS信号传导相关的细胞途径,这已经被证明适用于天然CBD。此外,我们观察到,在将CBD同系物与抑制内源大麻素代谢酶活性的药物(如单酰基甘油酶[MAGL]抑制剂)或与诱导细胞应激途径激活的药物(如酯类12-肉豆蔻酸13-乙酸酯[PMA])结合使用时,生物活性显著增强。
表征谱图
-
氢谱1HNMR
-
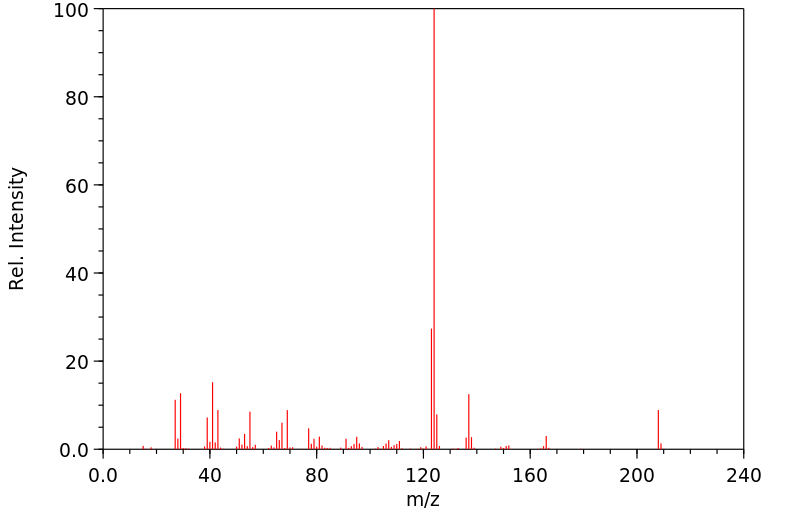
质谱MS
-
碳谱13CNMR
-
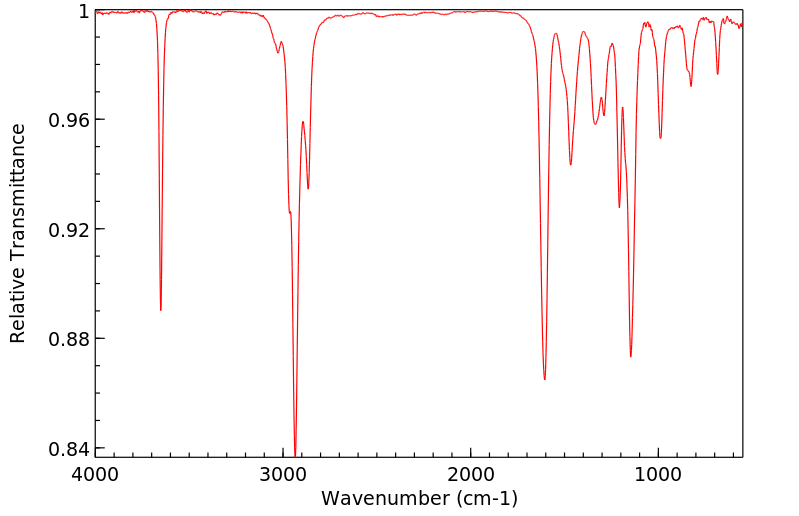
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息