1,4-二氯-2-丁醇 | 2419-74-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:90-95 °C(Press: 18 Torr)
-
密度:1,29 g/cm3
-
稳定性/保质期:
- 常温常压下稳定,需避免氧化物和光接触。
- 有毒且具有腐蚀性,可刺激黏膜和皮肤。生产设备应保持密闭以防泄漏,并要求操作人员佩戴防护用具以防止直接接触。参见β,β,β-三氯叔丁醇。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905590090
-
储存条件:应避光密封保存,置于阴凉、通风处。使用铁罐包装,每罐1kg,并内衬聚乙烯袋。请避开火源和高温环境,并按照易燃有毒化学品的规定进行储运。
SDS
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,4-Dichloro-2-butanol
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 4
Acute toxicity (Dermal)
Acute toxicity (Inhalation) Category 4
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Harmful if swallowed, in contact with skin or if inhaled
Hazard statements
Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
1,4-Dichloro-2-butanol
Section 2. HAZARDS IDENTIFICATION
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,4-Dichloro-2-butanol
>97.0%(GC)
Percent:
CAS Number: 2419-74-1
Chemical Formula: C4H8Cl2O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Rinse cautiously with water for several minutes. Remove contact lenses, if present
Eye contact:
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Unsuitable extinguishing Solid streams of water
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
from the chemical:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
1,4-Dichloro-2-butanol
Section 7. HANDLING AND STORAGE
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Form: Clear
Colour: Colorless - Slightly pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
1.29
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
1,4-Dichloro-2-butanol
Section 12. ECOLOGICAL INFORMATION
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
这是一种无色透明或浅棕色透明的液体,含量在80%-90%,沸程为120.2-135℃(12.666-13.332kPa),折射率为1.4882。该物质易溶于乙醇、乙醚和苯,而不溶于水。见光容易变色,因此需要避光保存。
用途
它主要用于医药中间体的制备。
生产方法
首先将1,2,4-丁三醇和冰乙酸加入反应锅内,并搅拌加热至约100℃。随后通入干燥的氯化氢气体,在90-110℃下反应约0.5小时,直到尾气开始出现大量氯化氢时检查反应终点并停止通氯化氢。冷却后用饱和碳酸钠溶液洗涤,再通过减压蒸馏收集在120-135℃(12.6-13.3kPa)之间的馏分,以得到油状的1,4-二氯-2-丁醇。收率约为50%-60%。原料消耗定额为:每吨1,2,4-丁三醇需要2965kg、冰乙酸50kg以及工业盐酸7000kg。
反应信息
-
作为反应物:描述:1,4-二氯-2-丁醇 在 三氟乙酸 作用下, 生成参考文献:名称:Synthesis of Precursors to Tetrazole Monomers by Acid-Catalyzed Alkylation摘要:The alkylation of 1H-tetrazole and 5-methyl-1H-tetrazole with 2-chloro- and 2-bromoethanol in concentrated sulfuric acid gave precursors to 2-ethenyl-2H-tetrazole and 2-ethenyl-5-methyl-2H-tetrazole in preparative yields. The alkylation process in deuterated sulfuric acid was studied by(1)H NMR spectroscopy. The yields of the alkylation products ranged from 14 to 76%, depending on the conditions and reactants, and the concentration of the 2-substituted isomers was no less than 99%.DOI:10.1134/s1070428020070131
-
作为产物:描述:参考文献:名称:Lithium or Bromomagnesium 1,4- and 1,5-Dilithioalkan-2-yloxides: Preparation and Synthetic Applications摘要:将氯甲基2-或3-氯烷醇处理与丁基锂,然后与萘锂,或与溴化乙基镁处理后再与粉末状锂,分别会形成锂或溴镁二锂醚,这些物质代表了三阴离子物种。溴镁1,ω-二锂-2-醚会立即脱除溴化锂和氧化镁,生成ω-锂-1-烯烃。这些后者的有机金属化合物以及锂二锂醚与各种电亲体反应,生成功能化的化合物。DOI:10.1055/s-1985-31412
文献信息
-
3-(5-HYDROXY-1-OXOISOINDOLIN-2-YL)PIPERIDINE-2,6-DIONE DERIVATIVES AND USES THEREOF申请人:Novartis AG公开号:US20200017461A1公开(公告)日:2020-01-16The present disclosure provides a compound of Formula (I′): or a pharmaceutically acceptable salt, hydrate, solvate, prodrug, stereoisomer, or tautomer thereof, wherein R x , X 1 , X 2 , and R 1 are as defined herein, and methods of making and using same.本公开提供了化合物Formula (I′)的一种: 或其药用可接受的盐、水合物、溶剂合物、前药、立体异构体或互变异构体,其中R x 、X 1 、X 2 和R 1 如本文所定义,并提供了制备和使用该化合物的方法。
-
2-(2-substituted申请人:A. H. Robins Company, Incorporated公开号:US05109013A1公开(公告)日:1992-04-28Novel compounds of the formula: ##STR1## wherein R and R.sup.1 are C.sub.1 -C.sub.6 alkyl or cycloalkyl and R.sup.2 and R.sup.3 are independently selected from C.sub.1 -C.sub.6 alkyl or aryl or --NR.sup.2 R.sup.3 forms a heterocyclic group which may be further substituted are disclosed. These compounds were found to possess muscle relaxant properties.新化合物的化学式为:##STR1## 其中R和R.sup.1为C.sub.1 -C.sub.6烷基或环烷基,R.sup.2和R.sup.3分别选自C.sub.1 -C.sub.6烷基或芳基或--NR.sup.2 R.sup.3形成的杂环基团,该基团可能进一步被取代。已经披露这些化合物具有肌肉松弛作用。
-
7-Substituted benzopyranes and process for the preparation thereof申请人:Chinoin Gyogyszer es Vegyeszeti Termekek Gyara R.T.公开号:US04391821A1公开(公告)日:1983-07-05The present invention relates to novel 7-substituted benzopyranes of formula (I), wherein R.sub.1 and R.sub.2 are hydrogen, alkyl of from 1 to 6 carbon atoms, hydroxyalkyl, alkenyl, cycloalkyl, phenylalkyl, dimethoxy-phenylalkyl, or R.sub.1 and R.sub.2 together with the joining nitrogen atom may represent a 5-7-membered heterocyclic ring, R.sub.3 is hydrogen, alkyl of from 1 to 4 carbon atoms or phenyl, R.sub.4 is hydrogen, R.sub.5 is hydrogen or phenyl, or R.sub.4 and R.sub.5 together may represent a bonding electron pair between the second and the third carbon atoms of the benzopyrane ring, R.sub.6 and R.sub.7 are hydrogen or they may represent an oxygen atom together, n is 1 or 2, with the proviso, that the pyrane ring may bear only one alkyl or phenyl substituent, and preparation process thereof. The novel compounds have valuable therapeutical effects, mainly in cardiotherapy.本发明涉及新型7-取代苯并吡喃母核化合物,其化学式为(I),其中R.sub.1和R.sub.2是氢,碳原子数为1至6的烷基,羟烷基,烯丙基,环烷基,苯甲基,二甲氧基苯甲基,或者R.sub.1和R.sub.2与连接的氮原子一起可以表示一个5-7成员的杂环,R.sub.3是氢,碳原子数为1至4的烷基或苯基,R.sub.4是氢,R.sub.5是氢或苯基,或者R.sub.4和R.sub.5一起可以表示苯并吡喃环的第二和第三碳原子之间的一个键合电子对,R.sub.6和R.sub.7是氢,或者它们可以一起表示一个氧原子,n是1或2,前提是吡喃环只能带有一个烷基或苯基取代基,以及其制备过程。这些新型化合物具有宝贵的治疗作用,主要在心脏治疗方面。
-
N-取代基-3-羟基四氢吡咯的制备方法申请人:常州市阳光药业有限公司公开号:CN106631956A公开(公告)日:2017-05-10
-
Preparation of<i>bis</i>‐quaternary ammonium salts from epichlorohydrin作者:Tae‐Seong Kim、Toshikazu Hirao、Isao IkedaDOI:10.1007/bf02523450日期:1996.1
Abstract A novel
bis ‐quaternary ammonium salt was prepared conveniently and almost quantitatively fromN,N ‐dimethyldodecylamine, its hydrochloride, and epichlorohydrin. Reaction ofN,N ‐dimethyldodecylamine with epichlorohydrin (in the presence of the amine hydrochloride) or various dichloro compounds was investigated by using1H nuclear magnetic resonance. The reaction route was studied by examining the reactivity of reagents with the amine and the effect of reaction temperature. The ease of the reaction with epichlorohydrin was found to be due to the assistance of amine hydrochloride in opening the epoxide ring and to neighboring‐group participation by the hydroxyl group of the intermediatemono ‐ammonium salt in the quaternization step. Neighboring‐group participation by the hydroxyl group in these quaternization reactions is also discussed.摘要 用 N,N-二甲基十二胺、其盐酸盐和环氧氯丙烷方便地制备了一种新型双季铵盐,且几乎定量。利用 1H 核磁共振研究了 N,N-二甲基十二胺与环氧氯丙烷(在胺盐酸盐存在下)或各种二氯化合物的反应。通过检测试剂与胺的反应性和反应温度的影响,研究了反应路线。研究发现,与环氧氯丙烷的反应之所以容易进行,是因为胺盐酸盐在打开环氧环时提供了帮助,而且中间单铵盐的羟基在季铵化步骤中参与了邻位基团。本文还讨论了羟基在这些季铵化反应中的邻基团参与。
表征谱图
-
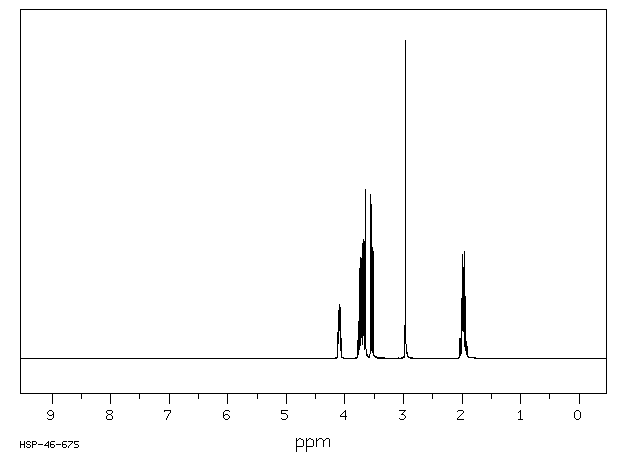
氢谱1HNMR
-
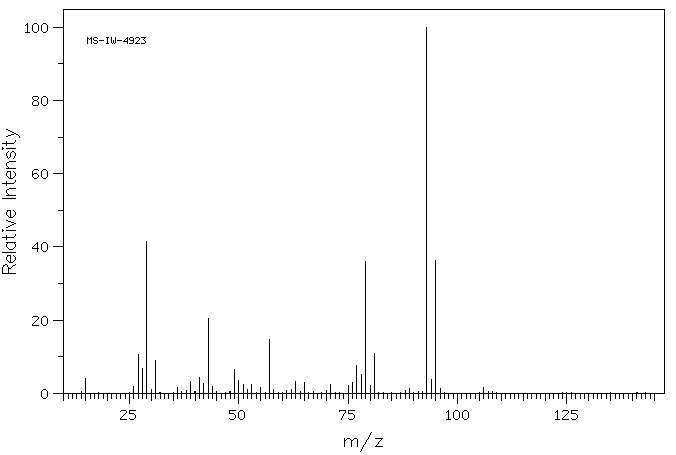
质谱MS
-
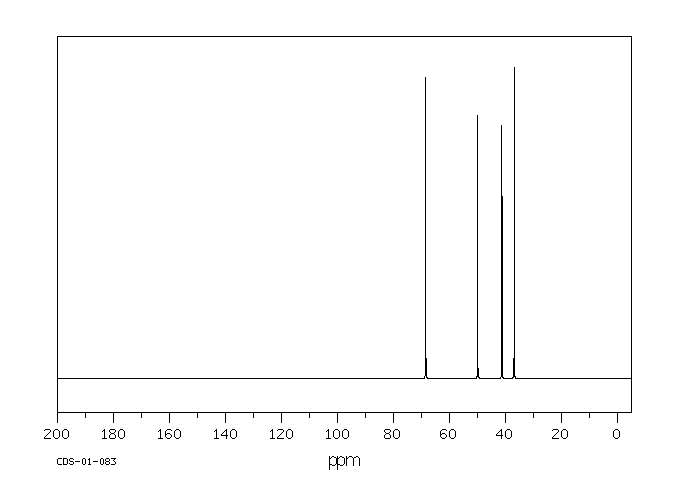
碳谱13CNMR
-
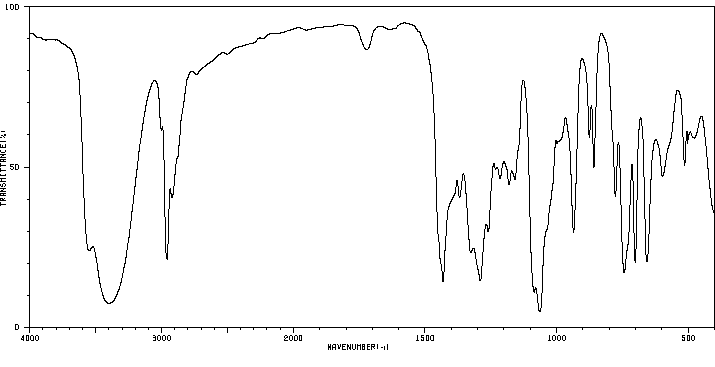
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息