苯氧乙酰肼 | 4664-55-5
中文名称
苯氧乙酰肼
中文别名
2-苯氧基乙酰肼
英文名称
phenoxyacetyl hydrazine
英文别名
phenoxyacetic acid hydrazide;phenoxyacetohydrazide;2-phenoxyacetohydrazide;phenoxyacetyl hydrazide
CAS
4664-55-5
化学式
C8H10N2O2
mdl
MFCD00297030
分子量
166.18
InChiKey
XSONSBDQIFBIOY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:112 °C
-
沸点:390.5±25.0 °C(Predicted)
-
密度:1.190±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:12
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:64.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2928000090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:应存于室温、密封、干燥、避光的环境中。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Phenoxyacetohydrazide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Phenoxyacetohydrazide
CAS number: 4664-55-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H10N2O2
Molecular weight: 166.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Phenoxyacetohydrazide
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-Phenoxyacetohydrazide
CAS number: 4664-55-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H10N2O2
Molecular weight: 166.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯氧乙酸 2-phenoxyacetic acid 122-59-8 C8H8O3 152.15 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 苯氧乙酰胺 phenoxyacetamide 621-88-5 C8H9NO2 151.165 —— N'-Acetyl-phenoxy-essigsaeure-hydrazid 51264-22-3 C10H12N2O3 208.217 —— 2-phenoxy-N'-(2-phenoxyacetyl)-acetohydrazide 53961-95-8 C16H16N2O4 300.314 —— N-phenoxyacetyl-N'-thiocarbamoyl-hydrazine 35687-21-9 C9H11N3O2S 225.271 —— 2-(4-chlorophenoxy)-N'-(2-phenoxyacetyl)acetohydrazide —— C16H15ClN2O4 334.759 —— N-methyl-2-(phenoxyacetyl)hydrazinecarbothioamide 35687-20-8 C10H13N3O2S 239.298 —— 1-phenoxyacetyl-2-benzylidenehydrazine 52093-72-8 C15H14N2O2 254.288 —— phenoxy-acetic acid cyclopentylidenehydrazide —— C13H16N2O2 232.282 —— 4-Oxo-4-[2-(2-phenoxyacetyl)hydrazinyl]but-2-enoic acid —— C12H12N2O5 264.238 —— N-[(4-methylphenyl)methylideneamino]-2-phenoxyacetamide —— C16H16N2O2 268.315 —— phenoxy-acetic acid cyclohexylidenehydrazide 157063-62-2 C14H18N2O2 246.309 苯氧乙酸 2-phenoxyacetic acid 122-59-8 C8H8O3 152.15 —— N-[(4-methoxyphenyl)methylideneamino]-2-phenoxyacetamide 201415-44-3 C16H16N2O3 284.315 —— N'-cycloheptylidene-2-phenoxyacetohydrazide —— C15H20N2O2 260.336 —— 1-phenoxyacetyl-2-cinnamylidenehydrazine —— C17H16N2O2 280.326 —— 1-phenoxyacetyl-2-(4-chlorobenzylidene)hydrazine 200725-90-2 C15H13ClN2O2 288.733 —— (E)-N′-(4-fluorobenzylidene)-2-phenoxyacetohydrazide —— C15H13FN2O2 272.279 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Grekov,A.P. et al., Journal of Organic Chemistry USSR (English Translation), 1970, vol. 6, # 1, p. 93 - 96摘要:DOI:
-
作为产物:参考文献:名称:通过双曼尼希型反应有效地一锅合成含手性侧链的s-三唑并[3,4- b ]-[1,3,5]噻二嗪摘要:通过单锅双曼尼希型反应开发了一种高效简便的方法,用于合成重要的手性s-三唑衍生物:(S)-3-α-苯基乙基-2,4-二氢-5-芳基-氧甲基-1,2,4-三唑[3,4- b ] -1,3,5-噻二嗪。DOI:10.1002/jhet.5570380418
文献信息
-
A new synthesis of phthalimidines作者:L. Yu. Ukhin、K. Yu. Suponitsky、L. V. Belousova、Zh. I. OrlovaDOI:10.1007/s11172-009-0347-1日期:2009.12A new synthesis of phthalimidines is described. 3-Acyloxy-2-aryl- and 2-acylamino-3-acyloxyphthalimidines were prepared by the reaction of 3-arylaminophthalides or o-formylbenzoic acid acylhydrazones with acetic or propionic anhydrides. Their reactions with O-, N-, S-, and C-nucleophiles were studied. The structure of 2-acetyl(cyanoacetyl)amino-3-acetoxyindolin-1-one was confirmed by X-ray diffraction
-
Synthesis and in-vitro antimicrobial activity of new 1,2,4-triazoles作者:A R Bhat、G Varadaraj Bhat、G Gautham ShenoyDOI:10.1211/0022357011775307日期:2010.2.18described the synthesis of new 1,2,4-triazoles and have evaluated their antimicrobial profile. Antitubercular activity was determined in triplicate using the Lowenstein-Jensen medium. A loopful of Mycobacterium tuberculosis suspension was inoculated on the surface of each Lowenstein-Jensen media containing the test compounds (100, 10 or 1 microg mL(-1)). To evaluate in-vitro antibacterial activity, compounds我们已经描述了新的1,2,4-三唑的合成,并评估了它们的抗菌特性。使用Lowenstein-Jensen培养基一式三份确定抗结核活性。在含有测试化合物(100、10或1微克mL(-1))的每种Lowenstein-Jensen培养基的表面上接种一圈结核分枝杆菌悬液。为了评估体外抗菌活性,通过圆盘扩散法对枯草芽孢杆菌,大肠杆菌,铜绿假单胞菌,金黄色葡萄球菌和伤寒葡萄球菌评估了化合物(50、5或0.5微克)。为了评估抗真菌活性,使用了Sabourauds葡萄糖琼脂培养基。筛选了某些化合物(5、0.5或0.05微克mL(-1))的抗黑曲霉88和黑曲霉90的活性,而其他化合物则抗T的活性。使用杯板法将红花TR1,红花R.R6,红花R7和薄荷茶T. 我们的结果表明,与在分子4位具有吡嗪部分的三唑相比,在3位具有吡嗪部分的三唑作为抗结核剂和抗真菌剂更具活性。
-
Design & synthesis of 2-(substituted aryloxy)-5-(substituted benzylidene)-3-phenyl-2,5-dihydro-1H-[1,2,4] triazin-6-one as potential anticonvulsant agents作者:Darpan Kaushik、Suroor Ahmad Khan、Gita ChawlaDOI:10.1016/j.ejmech.2010.05.051日期:2010.9A series of 2-(substituted aryloxy)-5-(substituted benzylidene)-3-phenyl-2,5-dihydro-1H-[1,2,4] triazin-6-one were designed & synthesized using appropriate synthetic route keeping in view the structural requirement of pharmacophore and evaluated for anticonvulsant activity and CNS activities. After intraperitoneal injection to mice, some synthesized derivatives were examined in the maximal electroshock设计并合成了一系列2-(取代的芳氧基)-5-(取代的亚苄基)-3-苯基-2,5-二氢-1 H- [1,2,4]三嗪-6-一。考虑到药效团的结构要求,并评估其抗惊厥活性和CNS活性。腹膜内注射给小鼠后,在最大的电击惊厥(MES)和皮下戊四氮(scPTZ)诱发的惊厥和神经毒性筛查中检查了一些合成的衍生物。还对那些发现有力的人进行了行为障碍和抑郁活动的评估。在测试的化合物中,两种动物模型中的5 eIX在剂量水平为30 mg / kg时均显示出对癫痫发作的保护作用,而5 bII和5 cII在相同剂量水平下显示出对scPTZ模型的保护作用。与临床有效药物相比,某些标题化合物的中枢神经系统抑制作用和神经毒性较小。
-
Molecular properties prediction and synthesis of novel 1,3,4-oxadiazole analogues as potent antimicrobial and antitubercular agents作者:Mohamed Jawed Ahsan、Jeyabalan Govinda Samy、Habibullah Khalilullah、Md. Shivli Nomani、Pankaj Saraswat、Ramakant Gaur、Abhimanyu SinghDOI:10.1016/j.bmcl.2011.10.057日期:2011.124-oxadiazol-2-yl)methyl]amino}-1,2-dihydro-3H-pyrazol-3-one were subjected to molecular properties prediction, drug-likeness by Molinspiration (Molinspiration, 2008) and MolSoft (MolSoft, 2007) software, lipophilicity and solubility parameters using ALOGPS 2.1 program. The compounds followed the Lipinski ‘Rule of five’ were synthesized for antimicrobial and antitubercular screening as oral bioavailable drugs/leads在本研究中,一系列1,5-二甲基-2-苯基-4-[((5-芳基-1,3,4-恶二唑-2-基)甲基]氨基]氨基} -1,2-二氢-使用ALOGPS 2.1程序,通过Molinspiration(Molinspiration,2008)和MolSoft(MolSoft,2007)软件对3 H-吡唑-3-酮进行分子性质预测,药物相似性,亲脂性和溶解度参数。合成了遵循Lipinski“ 5条规则”的化合物,作为口服生物可利用的药物/导线用于抗菌和抗结核病筛查。发现化合物4a的最大药物相似模型得分(0.95)。所有合成的化合物通过IR,NMR和质谱分析进行表征,然后进行抗菌和抗分枝杆菌筛选。在标题化合物中,化合物4d分别显示出对结核分枝杆菌H 37 Rv和耐异烟肼结核分枝杆菌(INHR-TB)的显着活性,最低抑菌浓度(MIC)分别为0.78μM和1.52μM。与标准药物环丙沙星相比,化合物4a对MIC
-
[EN] ALKYL SUBSTITUTED TRIAZOLE COMPOUNDS AS AGONISTS OF THE APJ RECEPTOR<br/>[FR] COMPOSÉS DE TRIAZOLE SUBSTITUÉS PAR ALKYLE EN TANT QU'AGONISTES DU RÉCEPTEUR APJ申请人:AMGEN INC公开号:WO2018093576A1公开(公告)日:2018-05-24Compounds of Formula I and Formula II, pharmaceutically acceptable salt thereof, stereoisomers of any of the foregoing, or mixtures thereof are agonists of the APJ Receptor and may have use in treating cardiovascular and other conditions. Compounds of Formula I and Formula II have the following structures: (I), (II) where the definitions of the variables are provided herein.化合物I和化合物II,其药用可接受的盐,上述任何一个的立体异构体,或它们的混合物是APJ受体的激动剂,可能用于治疗心血管和其他疾病。化合物I和化合物II具有以下结构:(I),(II),其中变量的定义在此提供。
表征谱图
-
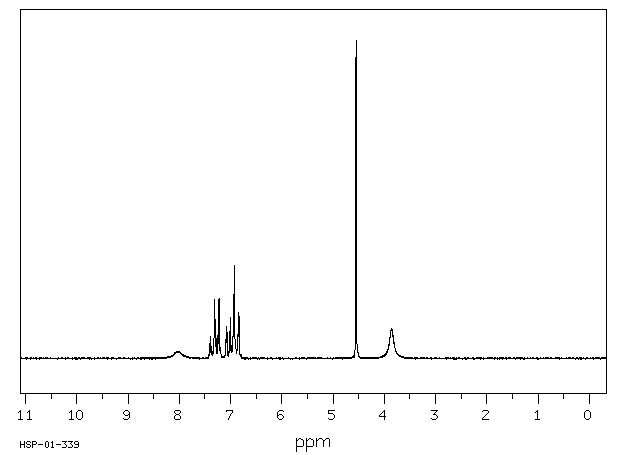
氢谱1HNMR
-
质谱MS
-
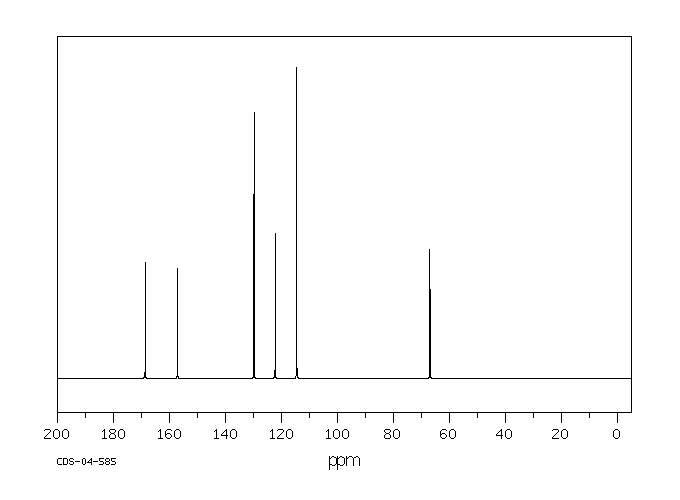
碳谱13CNMR
-
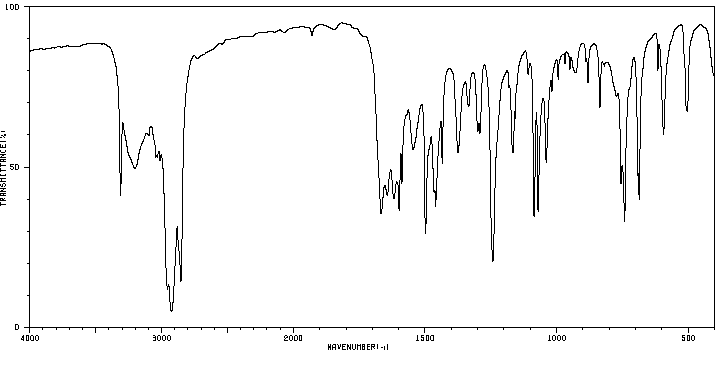
红外IR
-
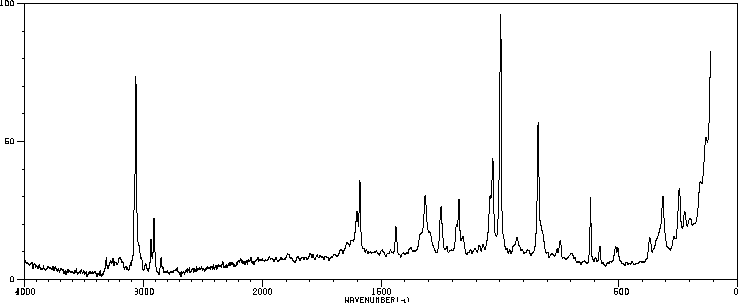
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯