1-(4-溴苯基)辛烷 | 51554-93-9
中文名称
1-(4-溴苯基)辛烷
中文别名
对溴辛基苯;1-溴-4-n-辛基苯;4-正辛基溴苯;1-溴-4-正辛基苯;1-溴-4-辛基苯;1-溴-4-N-辛基苯
英文名称
1-bromo-4-octylbenzene
英文别名
4-octylbromobenzene;1-bromo-4-n-octylbenzene;4-bromo(octylbenzene);4-n-octylbromobenzene;4-octyl-1-bromobenzene
CAS
51554-93-9
化学式
C14H21Br
mdl
——
分子量
269.225
InChiKey
OOZQSVXPBCINJF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:199-201°C
-
密度:1,13 g/cm3
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,目前没有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):6.4
-
重原子数:15
-
可旋转键数:7
-
环数:1.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
海关编码:2903999090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封保存在阴凉干燥处,并确保工作环境中具有良好通风或排气设施。
SDS
| Name: | 1-(4-Bromophenyl)octane 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 51554-93-9 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 51554-93-9 | 1-(4-Bromophenyl)octane | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 51554-93-9: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless - pale yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H21Br
Molecular Weight: 269.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, hydrogen bromide, bromine.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 51554-93-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(4-Bromophenyl)octane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 51554-93-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 51554-93-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 51554-93-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苯基辛烷 Octylbenzene 2189-60-8 C14H22 190.329 —— 1-(4-bromophenyl)octan-1-ol 76287-55-3 C14H21BrO 285.224 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-(4-溴苯基)-1-辛酮 1-(4-Bromophenyl)-1-octanone 7295-48-9 C14H19BrO 283.208 4-辛基苯乙炔 1-ethynyl-4-octylbenzene 79887-13-1 C16H22 214.351 4-正辛基苯乙烯 4-octylstyrene 46745-66-8 C16H24 216.367 对-辛基苯基氰 4-octylbenzonitrile 60484-68-6 C15H21N 215.338 —— (S)-3-(4-bromophenyl)cyclopentanone 160678-59-1 C11H11BrO 239.112 —— bis(4-octylphenyl)acetylene 913729-42-7 C30H42 402.663 —— (E)-3-(4-octylphenyl)acrylaldehyde —— C17H24O 244.377
反应信息
-
作为反应物:描述:参考文献:名称:对二硫代氨基甲酸酯前体路线的更深刻了解:用于光伏应用的可溶性聚亚苯基亚乙烯基衍生物的合成摘要:描述了两种新的聚亚噻吩乙烯撑衍生物的合成,即聚(3-辛基-2,5-噻吩撑乙烯撑)(O-PTV)和聚(双[辛基苯基-2,5-噻吩撑乙烯撑])( BOP-PTV)。两种聚合物均通过二硫代氨基甲酸酯(DTC)前体路线制备。通过使用不同的碱已经优化了单体向前体聚合物的聚合方案,从而提高了聚合步骤的可重复性。通过引入烷基侧链保证了可加工性。最后,将前体聚合物转化为共轭聚合物,并通过UV / vis,IR,GPC和循环伏安法对其进行了全面表征。以PCBM为受体的块状异质结太阳能电池显示出有希望的功率转换效率,对于BOP-PTV为0.80%,对于O-PTV为0.92%。DOI:10.1021/ma102101e
-
作为产物:描述:HEPTYLMAGNESIUM BROMIDE 在 platinum(IV) oxide potassium hydrogensulfate 、 氢气 作用下, 以 乙醚 、 乙醇 为溶剂, 反应 2.0h, 生成 1-(4-溴苯基)辛烷参考文献:名称:Franks, Stephen; Hartley, Frank R., Journal of the Chemical Society. Perkin transactions I, 1980, p. 2233 - 2237摘要:DOI:
文献信息
-
Regioselective Halogenation of Arenes and Heterocycles in Hexafluoroisopropanol作者:Ren-Jin Tang、Thierry Milcent、Benoit CrousseDOI:10.1021/acs.joc.7b02920日期:2018.1.19halogenation of arenes and heterocycles with N-halosuccinimides in fluorinated alcohols is disclosed. Under mild condition reactions, a wide diversity of halogenated arenes are obtained in good yields with high regioselectivity. Additionally, the versatility of the method is demonstrated by the development of one-pot sequential halogenation and halogenation-Suzuki cross-coupling reactions.
-
Synthesis of Highly Functionalized Pyridines for Planar Polymers. Maximized π-Conjugation in Electron Deficient Macromolecules作者:Yuxing Yao、Jaydeep J. S. Lamba、James M. TourDOI:10.1021/ja972744r日期:1998.4.1capture of the bis(isocyanate) effects the high-yielding conversion of carbonyl moieties to the tert-butoxycarbonyl-protected aryldiamine. Lithium−halogen exchange on the protected diaminopyridine followed by stannylation affords the required dimetalated diamine monomer. Treatment of the pyridine(dibromodiacid chloride) with mild cuprates or organocopper reagents affords the pyridine(dibromodiketones)
-
Phenyltellanyl Triflate (PhTeOTf) as a Powerful Tellurophilic Activator in the Friedel–Crafts Reaction作者:Takeshi Yamada、Eri Mishima、Kazuya Ueki、Shigeru YamagoDOI:10.1246/cl.2008.650日期:2008.6.5A powerful electrophilic activator for organotellurium compounds was developed. Phenyltellanyl triflate (PhTeOTf) prepared in situ from dibromophenyl(phenyltellanyl)telluride and AgOTf selectively activated various organotellurium compounds in the presence of aromatic compounds yielding the corresponding Friedel–Crafts reaction products. Polymer-end organotellurium compounds were also activated by PhTeOTf providing the corresponding end-functionalized polymers.
-
PHOTOBASE GENERATOR申请人:SAN-APRO LTD.公开号:US20160009737A1公开(公告)日:2016-01-14There is provided a photobase generator and a photosensitive resin composition containing the photobase generator. The photobase generator includes an ammonium salt represented by general formula (1). In formula (1), R 1 to R 4 independently represent an alkyl group having 1 to 18 carbon atoms or Ar, wherein at least one of R 1 to R 4 represents Ar; Ar represents an aryl group having 6 to 14 carbon atoms (excluding carbon atoms contained in a substituent as mentioned below), wherein some of hydrogen atoms in the aryl group may be independently substituted by an alkyl group having 1 to 18 carbon atoms or the like; Y + represents an ammonio group represented by general formula (2) or (3); and E represents a hydrogen atom or a group represented by general formula (5).
-
Method for producing biaryl compound申请人:SUMITOMO CHEMICAL COMPANY, LIMITED公开号:US20030158419A1公开(公告)日:2003-08-21There is disclosed a method for producing a biaryl compound of formula (I): 1 wherein R 1 is the same or different and independently denotes a substituted or unsubstituted hydrocarbon group or the like, A and B denote an aromatic hydrocarbon ring having from 6 to 14 carbon atoms or the like, k and m independently denote an integer of from 0 to 5, and 1 denotes an integer of 1 or 2, which method is characterized by reacting an aromatic compound of formula (II): 2 wherein R 1 , k and l denote the same as defined above, and X 1 denotes a leaving group, with a Grignard reagent of formula (III): 3 (R 2 ). 9MgX 2 (IIIg) wherein R 2 , B, and m denote the same as defined above and X 2 denotes chlorine or the like, in the presence of a cyclic ether, or an acyclic ether having two or more ether oxygens in the molecule and a nickel catalyst.
表征谱图
-
氢谱1HNMR
-
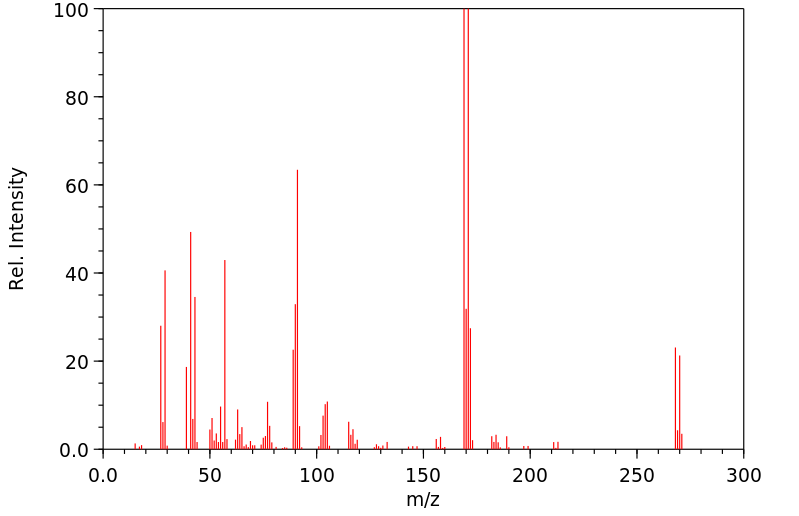
质谱MS
-
碳谱13CNMR
-
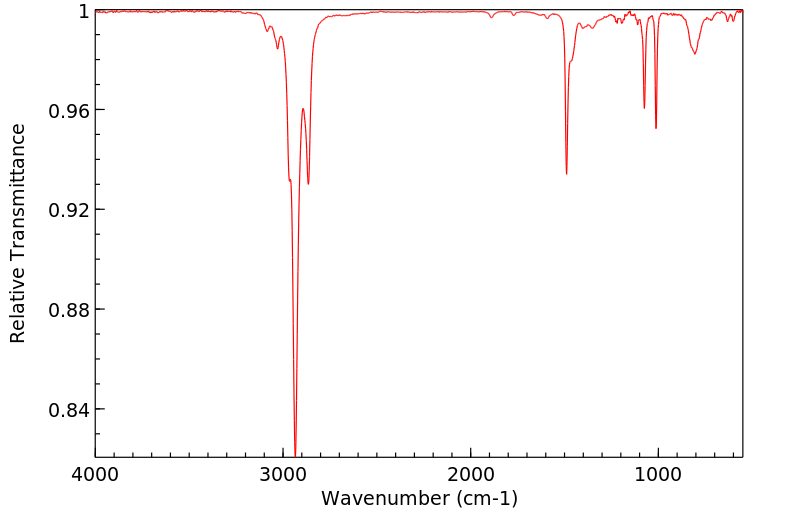
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫