3,4-二甲基苯甲醇 | 6966-10-5
中文名称
3,4-二甲基苯甲醇
中文别名
3,4-二甲基苄醇
英文名称
3,4-dimethylbenzyl alcohol
英文别名
(3,4-dimethylphenyl)methanol;(3,4-dimethylphenyl)methyl alcohol
CAS
6966-10-5
化学式
C9H12O
mdl
——
分子量
136.194
InChiKey
OKGZCXPDJKKZAP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:62-65 °C (lit.)
-
沸点:218-221 °C (lit.)
-
密度:0.9612 (rough estimate)
-
闪点:218-221°C
-
LogP:2.049 (est)
-
保留指数:1113
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S22,S26,S36/37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2906299090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
SDS
| Name: | 3 4-Dimethylbenzyl alcohol 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 6966-10-5 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6966-10-5 | 3,4-dimethylbenzyl alcohol | 99 | 230-175-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6966-10-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 218.0 - 221.0 deg C
Freezing/Melting Point: 62.00 - 65.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H12O
Molecular Weight: 136.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Acid chlorides, acid anhydrides, acids, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6966-10-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3,4-dimethylbenzyl alcohol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 6966-10-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6966-10-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6966-10-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲基苯甲酸 3,4-dimethyl benzoic acid 619-04-5 C9H10O2 150.177 1,2,4-三甲基苯 1,2,4-Trimethylbenzene 95-63-6 C9H12 120.194 3,4-二甲基苯甲酸甲酯 methyl 3,4-dimethylbenzoate 38404-42-1 C10H12O2 164.204 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3,4-二甲基苯甲酸 3,4-dimethyl benzoic acid 619-04-5 C9H10O2 150.177 —— 3',4'-dimethylphenylmethyl chloroformate 1427182-07-7 C10H11ClO2 198.649 —— 2-((3,4-Dimethylbenzyl)oxy)acetic acid 90296-13-2 C11H14O3 194.23 —— (2-iodo-4,5-dimethylphenyl)methanol 851384-78-6 C9H11IO 262.09 3,4-二甲基苯甲酸甲酯 methyl 3,4-dimethylbenzoate 38404-42-1 C10H12O2 164.204
反应信息
-
作为反应物:描述:3,4-二甲基苯甲醇 在 palladium on activated charcoal 哌啶 、 吡啶 、 PPA 、 氢气 、 pyridinium chlorochromate 作用下, 以 1,4-二氧六环 、 二氯甲烷 为溶剂, 25.0~80.0 ℃ 、206.84 kPa 条件下, 反应 52.5h, 生成 6,7-二甲基-2,3-二氢-1H-茚-1-酮参考文献:名称:Aromatische Spirane, 20. Mitt.: Darstellung von dimethylsubstituierten 2-Carboxymethyl-indan-1-onen und Benzylchloriden als Synthone f�r Synthesen von di- bis tetramethylierten 2,2?-Spirobiindandionen摘要:The isomeric dimethyl methylbenzoates 5, obtained from the bromides via Grignard reactions with dimethylcarbonate, were reduced with LiAlH4 to the hydroxymethyl derivatives 6. The latter were then transformed both to the benzylchlorides 7 (with SOCl2) and to the aldehydes 8 (with pyridinium chlorochromate). Knoevenagel-Doebner reaction of 8 afforded the acrylic acids 9 which (after hydrogenation to 11) were cyclized to the desired indanones 12 with polyphosphoric acid. On the other hand, 12c and 12e were prepared from dimethyl 3-chloropropiophenone (14) by warming with sulfuric acid. After NaH-catalyzed reaction with dimethylcarbonate, the indanones 12 gave the ketoesters 15 which then could be hydrogenated to the indanes 16. All reactions proceeded with satisfactory to excellent yields (60-90%).DOI:10.1007/bf00807400
-
作为产物:描述:参考文献:名称:NMR studies of bond order in distorted aromatic systems摘要:DOI:10.1021/ja00177a013
-
作为试剂:描述:3,4-二甲基苯甲醇 、 3,4-二甲基苯甲醛 、 1,2,4-三甲基苯 、 在 1,2,4-三甲基苯 、 3,4-二甲基苯甲醇 作用下, 反应 13.0h, 以Virtually no 3,4-dimethylbenzoic acid was produced的产率得到3,4-二甲基苯甲酸参考文献:名称:Method for the oxidation of aromatic compounds摘要:本发明涉及使用重组二甲苯单加氧酶表达微生物在两相反应介质中进行芳香族化合物的生物催化氧化的方法。公开号:US07368267B2
文献信息
-
Substituted N, N-disubstituted diamino compounds useful for inhibiting cholesteryl ester transfer protein activity申请人:——公开号:US20020120011A1公开(公告)日:2002-08-29The invention relates to substituted polycyclic aryl and heteroaryl tertiary-heteroalkylamine compounds useful as inhibitors of cholesteryl ester transfer protein (CETP; plasma lipid transfer protein-I) and compounds, compositions and methods for treating atherosclerosis and other coronary artery diseases. Preferred tertiary-heteroalkylamine compounds are substituted N,N-disubstituted diamines. A preferred specific N,N-disubstituted diamine is the compound: 1
-
一种合成手性醇的方法
-
Hydrogenation of Esters to Alcohols with a Well-Defined Iron Complex作者:Svenja Werkmeister、Kathrin Junge、Bianca Wendt、Elisabetta Alberico、Haijun Jiao、Wolfgang Baumann、Henrik Junge、Fabrice Gallou、Matthias BellerDOI:10.1002/anie.201402542日期:2014.8.11We present the first base‐free Fe‐catalyzed ester reduction applying molecular hydrogen. Without any additives, a variety of carboxylic acid esters and lactones were hydrogenated with high efficiency. Computations reveal an outer‐sphere mechanism involving simultaneous hydrogen transfer from the iron center and the ligand. This assumption is supported by NMR experiments.
-
Synthesis of Dibenzyls by Nickel-Catalyzed Homocoupling of Benzyl Alcohols作者:Xing-Zhong Shu、Feng-Feng Pan、Peng Guo、Xiaochuang HuangDOI:10.1055/a-1467-2432日期:2021.9blocks that are widely used in organic synthesis, and they are typically prepared by the homocoupling of halides, organometallics, and ethers. Herein, we report an approach to this class of compounds using alcohols, which are more stable and readily available. The reaction proceeds via nickel-catalyzed and dimethyl oxalate assisted dynamic kinetic homocoupling of benzyl alcohols. Both primary and secondary
-
Iron-Catalyzed Reduction of Carboxylic Esters to Alcohols作者:Kathrin Junge、Bianca Wendt、Shaolin Zhou、Matthias BellerDOI:10.1002/ejoc.201300039日期:2013.4formed from Fe(stearate)2/NH2CH2CH2NH2 and polymethylhydrosiloxane was directly developed for the hydrosilylation of carboxylic acid esters to alcohols. The catalytic method exhibits broad substrate scope, including 20 aliphatic, aromatic, and heterocyclic esters. The corresponding alcohols are obtained in moderate to very good yields.
表征谱图
-
氢谱1HNMR
-
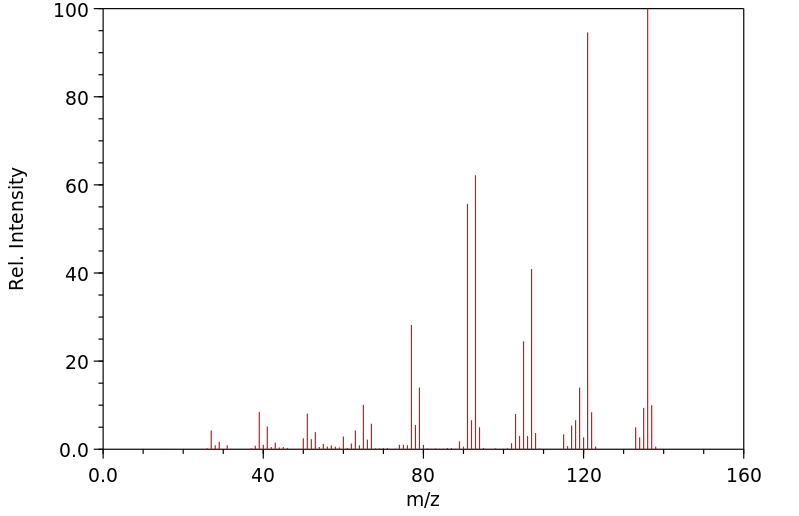
质谱MS
-
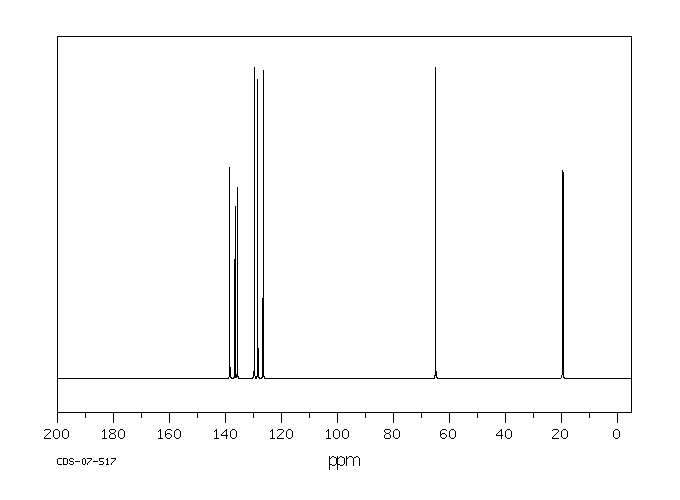
碳谱13CNMR
-
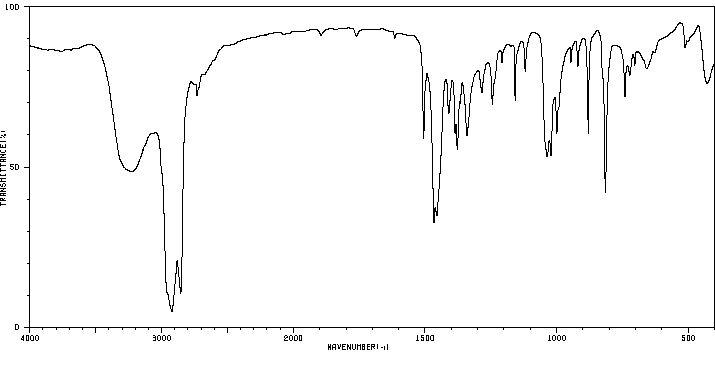
红外IR
-
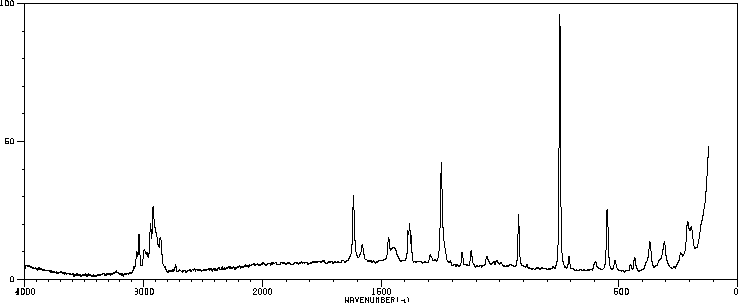
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫