三乙醇胺盐酸盐 | 637-39-8
物质功能分类
中文名称
三乙醇胺盐酸盐
中文别名
盐酸三乙醇胺(XZ);三乙醇胺-HCl;三乙醇銨鹽酸;2,2',2''-三羟基三乙胺盐酸盐;盐酸TEA盐;盐酸三乙醇胺;2''-次氮基三乙醇盐酸盐
英文名称
triethanolamine hydrochloride
英文别名
triethanolammonium chloride;triethanoloamine hydrochloride;2-[Bis(2-hydroxyethyl)amino]ethanol;hydron;chloride;2-[bis(2-hydroxyethyl)amino]ethanol;hydron;chloride
CAS
637-39-8
化学式
C6H16NO3*Cl
mdl
——
分子量
185.651
InChiKey
HHLJUSLZGFYWKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177-179 °C(lit.)
-
密度:1.354[at 20℃]
-
溶解度:H2O:1 Mat 20 °C,透明,无色
-
LogP:-3.697 at 20℃
-
物理描述:Triethanolamine hydrochloride is the salt of triethanolamine. See the chemical datasheet for triethanolamine for more information.
-
稳定性/保质期:
远离氧化物和水源。
计算性质
-
辛醇/水分配系数(LogP):-1.88
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:63.9
-
氢给体数:4
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:1
-
RTECS号:KL9346500
-
海关编码:2922132020
-
安全说明:S22,S24/25
-
储存条件:存放在密封容器内,并放置在阴凉、干燥处。储藏位置须远离氧化剂,避免接触湿气和水分。
SDS
| Name: | Ammonia Monitor Reagent Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 637-39-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 637-39-8 | Ethanol, 2,2',2''-nitrilotris-, hydroc | 1.59 | 211-284-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause severe eye irritation. May result in corneal injury.
May cause painful sensitization to light. May cause conjunctivitis.
Skin:
May cause skin irritation. May cause photosensitive skin reactions in certain individuals.
Ingestion:
May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation.
Chronic:
Prolonged or repeated skin contact may cause dermatitis.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Not flammable, but reacts with most metals to form flammable hydrogen gas. Use water spray to keep fire-exposed containers cool.
Extinguishing Media:
Substance is nonflammable; use agent most appropriate to extinguish surrounding fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Large spills may be neutralized with dilute alkaline solutions of soda ash (sodium carbonate, Na2CO3), or lime (calcium oxide, CaO).
Clean up spills immediately, observing precautions in the Protective Equipment section. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Keep away from heat and flame. Do not store in direct sunlight.
Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 637-39-8: CAS# 7732-18-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: 1.6
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Miscible with water.
Specific Gravity/Density: 1.01 (Water=1)
Molecular Formula: Mixture
Molecular Weight:
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, light.
Incompatibilities with Other Materials:
Acetate, acetic anhydride, alcohols + hydrogen cyanide, 2-aminoethanol, ammonium hydroxide, calcium carbide, calcium phosphide, cesium acetylene carbide, cesium carbide, chlorosulfonic acid, 1,1-difluoroethylene, ethylene diamine, ethyleneimine, fluorine, lithium silicide, magnesium boride, mercuric sulfate, oleum, perchloric acid, potassium permanganate, b-propiolactone, propylene oxide, rubidum acetylene carbide, rubidum carbide, silver perchlorate + carbon tetrachloride, sodium, sodium hydroxide, sulfuric acid, uranium phosphide, vinyl acetate. Substance polymerizes on contact with aldehydes or epoxides.
Hazardous Decomposition Products:
Hydrogen chloride, hydrogen gas.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 637-39-8: KL9346500 CAS# 7732-18-5: ZC0110000 LD50/LC50:
Not available.
CAS# 7732-18-5: Oral, rat: LD50 = >90 mL/kg.
Carcinogenicity:
Ethanol, 2,2',2''-nitrilotris-, hydrochloride - Not listed by ACGIH, IARC, or NTP.
Water - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Trout LC100=10 mg/L/24H Shrimp LC50=100-330 ppm Starfish LC50=100-330mg/L/48H Shore crab LC50=240 mg/L/48H Chronic plant toxicity=100 ppm
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 637-39-8: 1
CAS# 7732-18-5: No information available.
Canada
CAS# 637-39-8 is listed on Canada's DSL List.
CAS# 7732-18-5 is listed on Canada's DSL List.
CAS# 637-39-8 is not listed on Canada's Ingredient Disclosure List.
CAS# 7732-18-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 637-39-8 is listed on the TSCA inventory.
CAS# 7732-18-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:SOME NEW LOCAL ANESTHETICS CONTAINING THE MORPHOLINE RING摘要:DOI:10.1021/ja01358a049
-
作为产物:参考文献:名称:Structural study of the coordination behavior of a tetradentate NO3-donor amino alcohol ligand toward a CdII:HgII mixture摘要:
摘要 本文研究了2,2′,2″-硝基三乙醇(NTE)与CdI2和HgI2的1:1混合物的反应。合成并鉴定了复合物[Cd(NTE)2][Hg2(
μ -I)2I4],并通过元素分析、FT-IR、1H NMR光谱和单晶X射线衍射确定了其结构。还介绍了[HNTE]Cl盐的结构。在复合物的晶体结构中,镉原子具有CdN2O6的环境,呈略微扭曲的立方体几何形状。这种几何形状是镉配合物Cambridge结构数据库中罕见的最小扭曲之一。阴离子部分具有双核结构,其中汞原子处于四面体环境中。在复合物的网络中,除了O−H · · · I氢键外,还有I · · · I相互作用,导致了十元环。DOI:10.1515/znb-2016-0222 -
作为试剂:描述:isopropyl (ethoxy(4-nitrophenoxy)phosphoryl)-L-alaninate 在 三乙醇胺盐酸盐 、 S. cerevisiae carboxypeptidase Y 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 生成 (ethoxy(hydroxy)phosphoryl)-L-alanine参考文献:名称:ProTide 激活机制的区域化学分析摘要:ProTides 是用于治疗特定病毒感染的核苷酸类似物。这些化合物由掩蔽的核苷酸组成,该核苷酸在体内经历酶促和自发化学转化,产生游离的单核苷酸,最终转化为具有药用活性的三磷酸化药物。FDA 批准的三种 ProTides 由一个磷酸酰胺 (P-N) 核心与核苷类似物、苯酚和 l-丙烷基羧酸酯偶联组成。先前提出的活化机制假设存在不稳定的 5 元混合酸酐环状中间体,该中间体是由 l-丙烷基部分的羧酸盐基团的直接攻击与苯酚的排出形成的。进一步假设混合酸酐环状中间体发生自发水解,形成线性 l-丙氨酰氨基磷酸酯产物。在提出的激活机制中,先前已使用质谱法检测到 5 元混合酸酐中间体,但尚未确定水亲核攻击的特定位点(P-O 与 C-O)。为了进一步研究 ProTide 激活过程中形成的假定 5 元环状中间体的水解机制,使用可被羧肽酶 Y 激活的 ProTide 类似物在 18O 标记的水中进行反应。质谱和DOI:10.1021/acs.biochem.4c00176
文献信息
-
[EN] A CONJUGATE OF A CYTOTOXIC AGENT TO A CELL BINDING MOLECULE WITH BRANCHED LINKERS<br/>[FR] CONJUGUÉ D'UN AGENT CYTOTOXIQUE À UNE MOLÉCULE DE LIAISON CELLULAIRE AVEC DES LIEURS RAMIFIÉS申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2020257998A1公开(公告)日:2020-12-30Provided is a conjugation of cytotoxic drug to a cell-binding molecule with a side-chain linker. It provides side-chain linkage methods of making a conjugate of a cytotoxic molecule to a cell-binding ligand, as well as methods of using the conjugate in targeted treatment of cancer, infection and immunological disorders.提供了一种将细胞毒性药物与一个侧链连接分子结合的共轭物。它提供了制备细胞毒性分子与细胞结合配体的共轭物的侧链连接方法,以及在靶向治疗癌症、感染和免疫性疾病中使用该共轭物的方法。
-
[EN] CROSS-LINKED PYRROLOBENZODIAZEPINE DIMER (PBD) DERIVATIVE AND ITS CONJUGATES<br/>[FR] DÉRIVÉ DE DIMÈRE DE PYRROLOBENZODIAZÉPINE RÉTICULÉ (PBD) ET SES CONJUGUÉS申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2020006722A1公开(公告)日:2020-01-09A novel cross-linked cytotoxic agents, pyrrolobenzo-diazepine dimer (PBD) derivatives, and their conjugates to a cell-binding molecule, a method for preparation of the conjugates and the therapeutic use of the conjugates.
-
[EN] TYPE 4 PREPILIN PEPTIDASE INHIBITORS AND METHODS OF USE<br/>[FR] INHIBITEURS DE LA PRÉPILINE PEPTIDASE DE TYPE 4 ET PROCÉDÉS D'UTILISATION CORRESPONDANTS申请人:DARTMOUTH COLLEGE公开号:WO2015123130A1公开(公告)日:2015-08-20Compounds for inhibiting Type 4 Prepilin Peptidases are provided as are methods of using the compounds as anti-bacterial agents.抑制Type 4前体肽酶的化合物及其作为抗细菌剂的用途方法。
-
[EN] BIOCATALYTIC PROCESSES FOR THE PREPARATION OF VILANTEROL<br/>[FR] PROCÉDÉS BIOCATALYTIQUES DE PRÉPARATION DE VILANTÉROL申请人:TEVA PHARMACEUTICALS INT GMBH公开号:WO2017001907A1公开(公告)日:2017-01-05A process for preparing Vilanterol includes a biocatalytic conversion of a ketone substrate to its corresponding alcohol, and then converting the obtained alcohol into Vilanterol. Polypeptides may be used for the biocatalytic conversion of the ketone substrate, such as 2-bromo-l-(2,2-dimethyl-4H-l,3-benzodioxin-6-yl)ethanone, to an enantiopure alcohol, (R)-2-bromo-l-(2,2-dimethyl-4H-l,3-benzodioxin-6-yl)ethanol for the preparation of Vilanterol. Also disclosed is vilanterol tartrate and solid state forms thereof for use as medicaments and for the preparation of other vilanterol salts, or of vilanterol, solid state forms and/or formulations thereof. Also disclosed is a process for the preparation of pharmaceutical formulations including vilanterol tartrate and solid state forms thereof, as well as a method of treating a person suffering from COPD and asthma by administering a therapeutically effective amount of any one or a combination of vilanterol tartrate and solid state forms thereof or a pharmaceutical composition and/or formulation comprising vilanterol tartrate and solid state forms thereof.制备维兰特罗的过程包括将酮底物进行生物催化转化为其相应的醇,然后将得到的醇转化为维兰特罗。多肽可用于酮底物的生物催化转化,例如将2-溴-1-(2,2-二甲基-4H-1,3-苯并二氧杂环己-6-基)乙酮转化为对映纯醇,(R)-2-溴-1-(2,2-二甲基-4H-1,3-苯并二氧杂环己-6-基)乙醇,用于制备维兰特罗。还公开了维兰特罗酒石酸盐及其作为药物用途的固态形式,以及用于制备其他维兰特罗盐或维兰特罗、固态形式和/或其制剂。还公开了一种包括维兰特罗酒石酸盐和固态形式的制备药物制剂的过程,以及通过给予治疗有效量的维兰特罗酒石酸盐和固态形式的任一种或组合,或包含维兰特罗酒石酸盐和固态形式的药物组合物和/或制剂,来治疗患有慢性阻塞性肺病(COPD)和哮喘的人的方法。
-
[EN] CONJUGATION LINKERS, CELL BINDING MOLECULE-DRUG CONJUGATES CONTAINING THE LIKERS, METHODS OF MAKING AND USES SUCH CONJUGATES WITH THE LINKERS<br/>[FR] LIEURS DE CONJUGAISON, CONJUGUÉS MÉDICAMENT-MOLÉCULE DE LIAISON À UNE CELLULE CONTENANT LESDITS LIEURS, PROCÉDÉS DE PRÉPARATION ET D'UTILISATION DE TELS CONJUGUÉS AVEC LES LIEURS申请人:HANGZHOU DAC BIOTECH CO LTD公开号:WO2018086139A1公开(公告)日:2018-05-17The present invention relates to linkers having a group of propiolyl, substituted acryl (acryloyl), or disubstituted propanoyl, and using such linkers for the conjugation of compounds, in particular, cytotoxic agents to a cell-binding molecule.
表征谱图
-
氢谱1HNMR
-
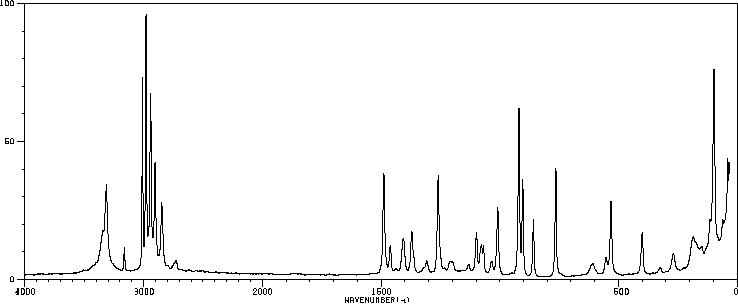
质谱MS
-
碳谱13CNMR
-
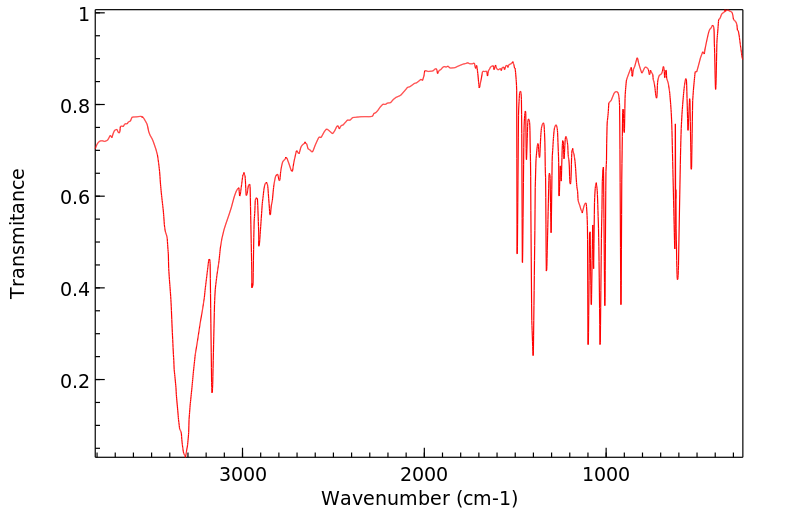
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷