环庚甲酸 | 1460-16-8
中文名称
环庚甲酸
中文别名
环庚烷羧酸;环庚基甲酸
英文名称
cycloheptanoic acid
英文别名
cycloheptane carboxylic acid;cycloheptylcarboxylic acid;Cycloheptanecarboxylic acid
CAS
1460-16-8
化学式
C8H14O2
mdl
MFCD00004152
分子量
142.198
InChiKey
VZFUCHSFHOYXIS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:97.5-98.0 °C
-
沸点:135-138 °C9 mm Hg(lit.)
-
密度:1.035 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.875
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2916209090
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:常温、避光、存于通风干燥处。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : Cycloheptanecarboxylic acid
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 1460-16-8
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
SECTION 2: Hazards identification
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008
This substance is not classified as dangerous according to Directive 67/548/EEC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Formula : C8H14O2
Molecular Weight : 142,20 g/mol
CAS-No. : 1460-16-8
EC-No. : 215-954-5
No components need to be disclosed according to the applicable regulations.
SECTION 4: First aid measures
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Avoid breathing vapours, mist or gas.
For personal protection see section 8.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Normal measures for preventive fire protection.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection not required. For nuisance exposures use type OV/AG (US) or type ABEK
(EU EN 14387) respirator cartridges. Use respirators and components tested and approved under
appropriate government standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Do not let product enter drains.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: liquid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 135 - 138 °C at 12 hPa - lit.
boiling range
g) Flash point > 113,00 °C - closed cup
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,035 g/mL at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: Not available
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
no data available
SECTION 13: Disposal considerations
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16: Other information
Further information
Copyright 2013 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
用途:有机合成
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙基环庚烷羧酸酯 ethyl cycloheptylcarboxylate 32777-26-7 C10H18O2 170.252 1-羟基-环庚羧酸 1-hydroxycycloheptanecarboxylic acid 20920-03-0 C8H14O3 158.197 环庚烷甲醇 cycloheptylmethanol 4448-75-3 C8H16O 128.214 环庚烷甲醛 cycloheptanecarboxaldehyde 4277-29-6 C8H14O 126.199 —— 4-cycloheptene-1-carboxylic acid 1614-73-9 C8H12O2 140.182 —— Cycloheptan-dicarbonsaeure-(1,1) 90612-83-2 C9H14O4 186.208 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-methyl-cycloheptanecarboxylic acid 35664-93-8 C9H16O2 156.225 甲基环庚烷羧酸 methyl cycloheptanecarboxylate 60433-00-3 C9H16O2 156.225 —— 1-decyl-1-cycloheptanecarboxylic acid 140113-01-5 C18H34O2 282.467 乙基环庚烷羧酸酯 ethyl cycloheptylcarboxylate 32777-26-7 C10H18O2 170.252 —— n-octyl cycloheptanecarboxylate 1180629-99-5 C16H30O2 254.413 1-羟基-环庚羧酸 1-hydroxycycloheptanecarboxylic acid 20920-03-0 C8H14O3 158.197 环庚烷甲醇 cycloheptylmethanol 4448-75-3 C8H16O 128.214 环庚烷甲醛 cycloheptanecarboxaldehyde 4277-29-6 C8H14O 126.199 —— cycloheptanecarboxylic acid anhydride —— C16H26O3 266.381 —— ethyl 4-oxocycloheptanecarboxylate 171361-62-9 C10H16O3 184.235
反应信息
-
作为反应物:描述:参考文献:名称:氧的开/关O2可切换光催化氧化和原脱羧。摘要:近年来,光氧化还原催化已成为有机合成中科学的强大分支。尽管将光氧化还原和金属催化剂合并已被广泛使用,但很少考虑可切换的多相光氧化还原催化。在这里,我们打开了一个新的窗口,以使用可切换的多相光氧化还原催化剂,该催化剂可以通过更改两个对手反应(即氧化和原脱羧)的简单刺激(O2)来打开/关闭。使用这种策略,我们证明Au @ ZnO核壳纳米粒子可以用作可转换的光催化剂,由于金的局部表面等离振子共振效应,它具有良好的吸收可见光的催化活性,可以使多种芳族和脂族脱羧羧酸,具有多种可重复使用性,并且是大规模合成醛/酮和烷烃/芳烃的合理候选者。还通过广泛提供药物的直接氧化和原脱羧的例子显示了一些生物活性分子。DOI:10.1021/acs.joc.9b01759
-
作为产物:参考文献:名称:环烷酮的氧化环缩合:一种合成中环环烷羧酸的简便方法摘要:摘要 环烷酮(1)在聚(双蒽基)二硒化物(5b)作为催化剂存在下,用30%的过氧化氢氧化,生成环中比底物少一个碳原子的环烷烃羧酸(2)。尽管酸 2 的制备产率不超过 60%,但该反应可作为合成具有五至七元环的环烷羧酸的简单方法。DOI:10.1080/00397919908086230
-
作为试剂:参考文献:名称:Imidazopyrazine tyrosine kinase inhibitors摘要:该式化合物及其药学上可接受的盐,其中Q1和R1的定义如本文所述,抑制IGF-1R酶并且适用于治疗和/或预防多种对酪氨酸激酶抑制治疗有反应的疾病和病况。公开号:US07459554B2
文献信息
-
5-arylpyrimidines as anticancer agents申请人:Zhang Nan公开号:US20050075357A1公开(公告)日:2005-04-07This invention relates to certain 5-arylpyrimidine compounds or a pharmaceutically acceptable salt thereof, and compositions containing said compounds or a pharmaceutically acceptable salt thereof, wherein said compounds are anti-cancer agents useful for the treatment of cancer in mammals. This invention further relates to a method of treating or inhibiting the growth of cancerous tumor cells and associated diseases in a mammal and further provides a method for the treatment or prevention of cancerous tumors that express multiple drug resistance (MDR) or are resistant because of MDR, in a mammal in need thereof which method comprises administering to said mammal an effective amount of said compounds or a pharmaceutically acceptable salt thereof. More specifically, the present invention relates to a method of treating or inhibiting the growth of cancerous tumor cells and associated diseases in a mammal in need thereof by promotion of microtubule polymerization which comprises administering to said mammal an effective amount of said compounds and pharmaceutically acceptable salts thereof.这项发明涉及某些5-芳基嘧啶化合物或其药用盐,以及含有所述化合物或其药用盐的组合物,其中所述化合物是抗癌剂,可用于治疗哺乳动物的癌症。该发明还涉及一种治疗或抑制哺乳动物体内癌细胞和相关疾病生长的方法,并进一步提供了一种治疗或预防表达多药耐药性(MDR)或因MDR耐药的癌症肿瘤的方法,该方法包括向所述哺乳动物体内施用所述化合物或其药用盐的有效量。更具体地,本发明涉及通过促进微管聚合来治疗或抑制哺乳动物体内癌细胞和相关疾病的方法,该方法包括向所述哺乳动物体内施用所述化合物及其药用盐的有效量。
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS AND USES THEREOF申请人:Florjancic S. Alan公开号:US20080058335A1公开(公告)日:2008-03-06The present invention relates to compounds of formula (I), or pharmaceutical salts, prodrugs, salts of prodrugs, or combinations thereof, wherein R 1 , R 2 , R 3 , and L 1 are defined in the specfication, compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions. The present invention also relates to compounds of formula (II), or pharmaceutical salts, prodrugs, salts of prodrugs, or combinations thereof, wherein R 1a , R 2a and (Rx)n are as defined in the specification, compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions.本发明涉及式(I)的化合物,或药用盐、前药、前药的盐或其组合物, 其中R 1 ,R 2 ,R 3 和L 1 在规范中定义,包含这种化合物的组合物,以及使用这种化合物和组合物治疗疾病和疾病的方法。本发明还涉及式(II)的化合物,或药用盐、前药、前药的盐或其组合物, 其中R 1a ,R 2a 和(Rx)n如规范中定义,包含这种化合物的组合物,以及使用这种化合物和组合物治疗疾病和疾病的方法。
-
Indol-3-ylcycloalkyl Ketones: Effects of N1 Substituted Indole Side Chain Variations on CB<sub>2</sub> Cannabinoid Receptor Activity作者:Jennifer M. Frost、Michael J. Dart、Karin R. Tietje、Tiffany R. Garrison、George K. Grayson、Anthony V. Daza、Odile F. El-Kouhen、Betty B. Yao、Gin C. Hsieh、Madhavi Pai、Chang Z. Zhu、Prasant Chandran、Michael D. MeyerDOI:10.1021/jm901214q日期:2010.1.14affinity CB2 agonists (5, 16). Substitution at the N1-indole position was then examined. A series of aminoalkylindoles was prepared and several substituted aminoethyl derivatives were active (23−27, 5) at the CB2 receptor. A study of N1 nonaromatic side chain variants provided potent agonists at the CB2 receptor (16, 35−41, 44−47, 49−54, and 57−58). Several polar side chains (alcohols, oxazolidinone) were已经制备了几种对CB 2大麻素受体具有高亲和力并且对CB 1受体具有选择性的3-酰基环吲哚。各种3-酰基取代基进行了调查,和四甲基环组被发现导致高亲和力CB 2激动剂(5,16)。然后检查在N1-吲哚位置的取代。(A系列aminoalkylindoles的制备和几个取代氨基乙基衍生物是活性23 - 27,5在CB)2受体。N1非芳香族侧链变异体的研究为CB 2受体提供了有效的激动剂(16,35 - 41,44 - 47,49 - 54,和57 - 58)。几个极性侧链(醇类,恶唑烷酮)的良好的耐受性为CB 2受体的活性(41,50),而其他(酰胺,酸)导致较弱的或无活性的化合物(55和56)。N1芳香族侧链还提供几个高亲和力CB 2受体激动剂(61,63,65,和69),但是在体外CB一般不太有效的2个功能测定法均高于非芳香族侧链类似物。
-
Photoredox-catalyzed decarboxylative alkylation/cyclization of alkynylphosphine oxides: a metal- and oxidant-free method for accessing benzo[<i>b</i>]phosphole oxides作者:Lixin Liu、Jianyu Dong、Yani Yan、Shuang-Feng Yin、Li-Biao Han、Yongbo ZhouDOI:10.1039/c8cc08689c日期:——
By photoredox-catalysis, alkylation/aryl cyclization of alkynylphosphine oxides towards benzo[
b ]phospholes has been realized under metal- and oxidant-free conditions at room temperature.通过光氧化催化,在室温无金属和氧化剂条件下,实现了炔膦氧化物向苯并[b]膦的烷基化/芳基环化。 -
Organophotoredox-Catalyzed Formation of Alkyl–Aryl and −Alkyl C–S/Se Bonds from Coupling of Redox-Active Esters with Thio/Selenosulfonates作者:Yue Dong、Peng Ji、Yueteng Zhang、Changqing Wang、Xiang Meng、Wei WangDOI:10.1021/acs.orglett.0c03624日期:2020.12.18A mild organophotoredox synthetic protocol for forming a Csp3–S/Se bond by reacting widespread redox-active esters with thio/selenosulfonates has been developed. The power of the synthetic manifold is fueled by an unprecedented broad substrate scope and wide functional group tolerance.
表征谱图
-
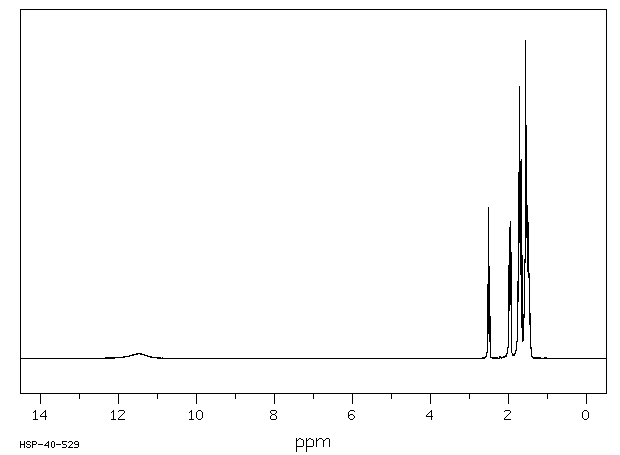
氢谱1HNMR
-
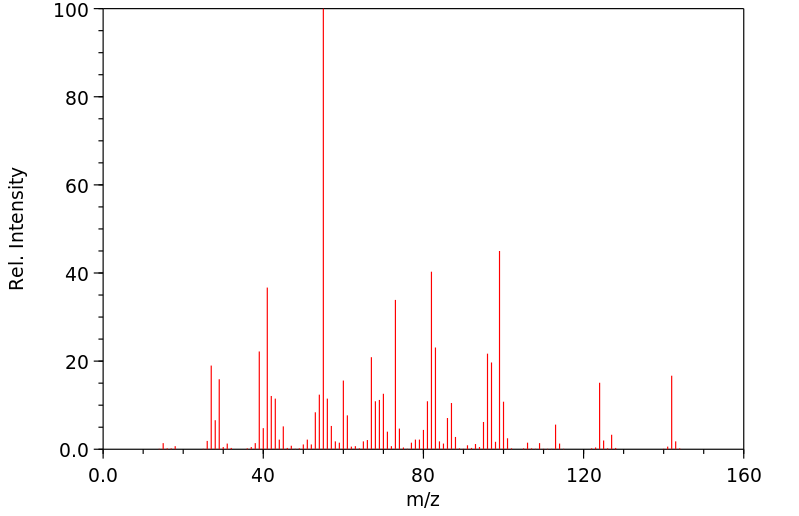
质谱MS
-
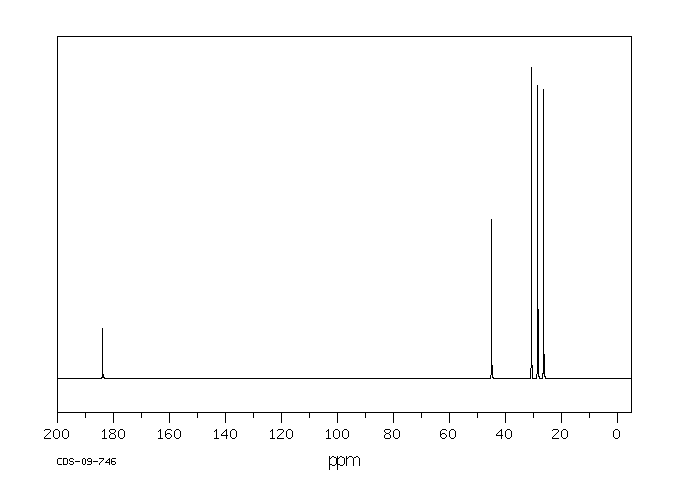
碳谱13CNMR
-
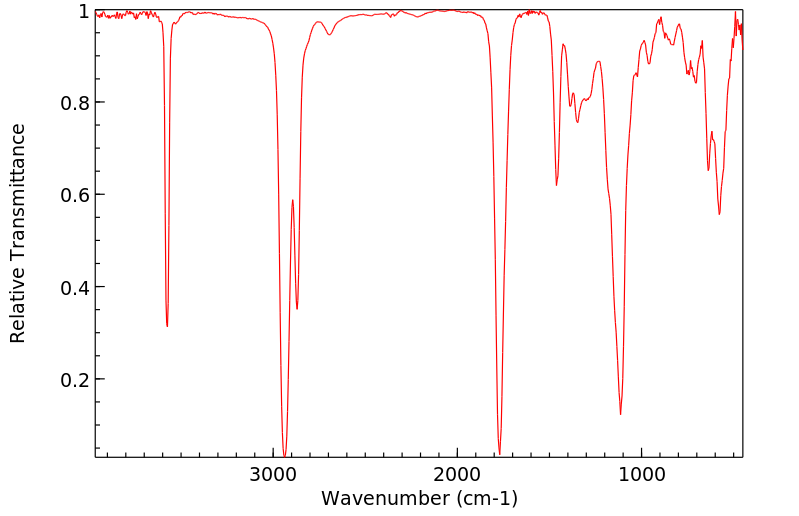
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸