烯丙亚基环己烷 | 5664-10-8
中文名称
烯丙亚基环己烷
中文别名
——
英文名称
allylidenecyclohexane
英文别名
prop-2-enylidenecyclohexane
CAS
5664-10-8
化学式
C9H14
mdl
MFCD00049192
分子量
122.21
InChiKey
OZHPVKGWTMAESF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:172.2±7.0 °C(Predicted)
-
密度:0.903±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-环己基亚基-乙醇 2-cyclohexylidene ethanol 932-89-8 C8H14O 126.199 环己基亚基乙醛 cyclohexylideneacetaldehyde 1713-63-9 C8H12O 124.183 环己乙炔 vinylidenecyclohexane 5664-20-0 C8H12 108.183
反应信息
-
作为反应物:参考文献:名称:Lewina; Trachtenberg, Zhurnal Obshchei Khimii, 1936, vol. 6, p. 764,771摘要:DOI:
-
作为产物:参考文献:名称:聚锂有机化合物— 23.通过将锂金属添加到1,4-非对称取代的丁烯中来形成3,4-二硫代-1,2-丁二烯摘要:描述了高反应性的1,4-不对称取代的丁烯12a-c的合成。当采用严格的合成方案时,这些链烷烃与锂金属反应生成3,4-二硫代-1,2-丁二烯20a-c,作为稳定的中间体。20的结构得到IR和NMR光谱证据的支持。在一种情况下,可以通过1,2-丁二烯19的双重去质子化反应来制备相同的双阴离子中间体。一旦衍生化,或者3,4-二取代的1,2-丁二烯24,2,3-二取代的1,3-丁二烯25,或1,4-二取代-2-丁炔26被形成,这取决于所用的亲电子试剂的性质。DOI:10.1016/0040-4020(96)00235-9
文献信息
-
Boron Lewis Acid-Catalyzed Regioselective Hydrothiolation of Conjugated Dienes with Thiols作者:Gautam Kumar、Zheng-Wang Qu、Soumen Ghosh、Stefan Grimme、Indranil ChatterjeeDOI:10.1021/acscatal.9b04647日期:2019.12.6tris(pentafluorophenyl)borane, B(C6F5)3, and BF3·Et2O are shown to catalyze the regioselective hydrothiolation of a wide range of terminal 1-aryl-1,3-dienes. In the case of internal 1,3-dienes, B(C6F5)3 is by far the better catalyst than BF3·Et2O. The process features mild reaction conditions, broad scope, and low catalyst loading, and it can be scaled up quickly over a short reaction time. The reactions are rate-limited
-
Organoselenium-Catalyzed Regioselective C−H Pyridination of 1,3-Dienes and Alkenes作者:Lihao Liao、Ruizhi Guo、Xiaodan ZhaoDOI:10.1002/anie.201610657日期:2017.3.13organoselenium‐catalyzed regioselective C−H pyridination of 1,3‐dienes to form pyridinium salts has been developed. This method was also successfully applied to direct C−H pyridination of alkenes. Fluoropyridinium reagents, or initially loaded pyridine derivatives, acted as pyridine sources in the pyridination reactions. The obtained pyridinium salts could be further converted under different conditions
-
Allylic Arylation of 1,3-Dienes via Hydroboration/Migrative Suzuki–Miyaura Cross-Coupling Reactions作者:Xiao-Ming Zhang、Jie Yang、Qing-Bo Zhuang、Yong-Qiang Tu、Zongyuan Chen、Hui Shao、Shao-Hua Wang、Fu-Min ZhangDOI:10.1021/acscatal.8b01823日期:2018.7.6The hydroboration/Pd-catalyzed migrative Suzuki–Miyaura cross-coupling of 1,3-dienes with electron-deficient aryl halides has been developed, which enables the synthesis of branched allylarenes directly from primary homoallylic alkyl boranes. A ligand-tuned linear- or branch-selective coupling for these aryl halides has also been achieved.
-
Interception of Nazarov Reactions of Allenyl Vinyl Ketones with Dienes: (3+2)- versus (4+3)-Cycloaddition and Subsequent Rearrangement作者:Timothy D. R. Morgan、François M. LeFort、Zhe Li、Vanessa M. Marx、Russell J. Boyd、D. Jean BurnellDOI:10.1002/ejoc.201403618日期:2015.5Capture of the cyclic oxyallyl cation intermediates from the BF3-mediated Nazarov reactions of three allenyl vinyl ketones with various dienes was accomplished by (3+2)- and (4+3)-cycloaddition. The relative amounts of these types of products were dependent on the substitution on the diene, and this could be linked to steric hindrance. Treatment of the (3+2)-cycloaddition products with BF3·Et2O led mainly
-
Catalytic Enantioselective 1,2-Diboration of 1,3-Dienes: Versatile Reagents for Stereoselective Allylation作者:Laura T. Kliman、Scott N. Mlynarski、Grace E. Ferris、James P. MorkenDOI:10.1002/anie.201105716日期:2012.1.9More with boron: The development of catalytic enantioselective 1,2‐diboration of 1,3‐dienes enables a new strategy for enantioselective carbonyl allylation reactions (see scheme). These reactions occur with outstanding levels of stereoselection and can be applied to both monosubstituted and 1,1‐disubstituted dienes. The carbonyl allylation reactions provide enantiomerically enriched functionalized
表征谱图
-
氢谱1HNMR
-
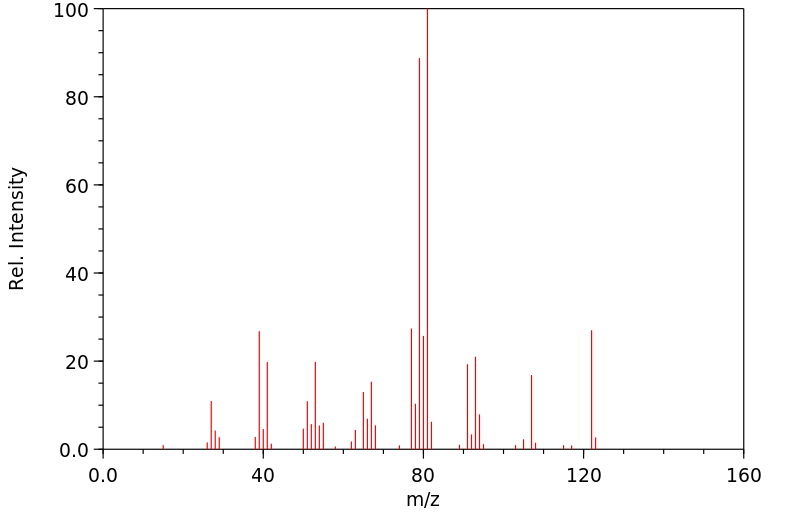
质谱MS
-
碳谱13CNMR
-
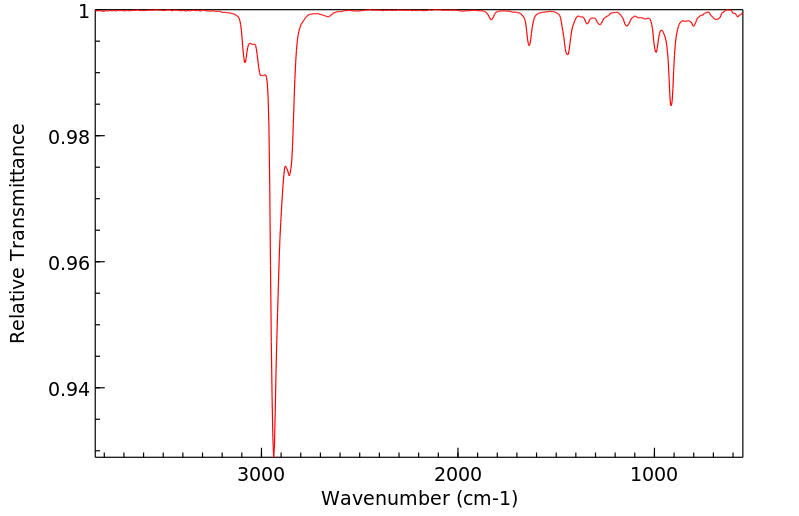
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-